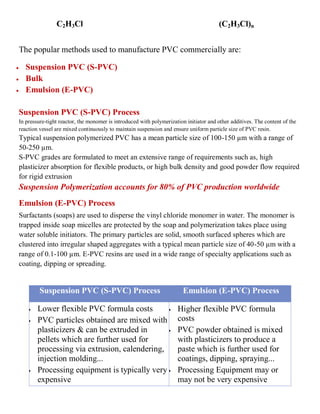
Polyvinyl chloride (PVC) is the third most widely produced synthetic plastic polymer. It exists in both rigid and flexible forms. Rigid PVC is used in construction for pipes and windows/doors, while flexible PVC is used in products like flooring, phonograph records, and medical tubing. PVC is produced by polymerizing vinyl chloride monomer and is a versatile material with many applications due to its low cost, chemical resistance, and flame retardant properties. It is commonly used in pipes, wire insulation, siding, and signs.
![POLYVINYL CHLORIDE
Polyvinyl chloride (PVC) is the world's third-most widely produced
synthetic plastic polymer (after polyethylene and polypropylene).
About 40 million tons of PVC are produced each year.
PVC comes in two basic forms:
I. Rigid (RPVC)
II. Flexible. (PPVC)
I. Rigid (RPVC or uPVC)
The rigid form of PVC is used in construction for pipe and in profile
applications such as doors and windows. It is also used in making bottles,
non-food packaging, food-covering sheets,[8]
and cards (such as bank or
membership cards).
II. Flexible (PPVC)
It can be made softer and more flexible by the addition of plasticizers, the
most widely used being phthalates. In this form, it is also used in plumbing,
electrical cable insulation, imitation leather, flooring,
signage, phonograph records, inflatable products, and many applications
where it replaces rubber. With cotton or linen, it is used in the production
of canvas.
Pure polyvinyl chloride is a white, brittle solid. It is insoluble in alcohol but
slightly soluble in tetrahydrofuran (THF).
Strengths Limitations
Rigid PVC
Low cost & high stiffness
Intrinsic flame retardant
FDA compliant & also suitable for
transparent applications
Better chemical resistance than plasticized
PVC
Good electrical insulation & vapor barrier
properties
Difficult to melt process
Limited solvent stress cracking
resistance
Becomes brittle at 5°C (when not
modified with impact modifiers
and/or processing aids)
Low continuous service
temperature of 50°C](https://image.slidesharecdn.com/pvcandvinylpolymers-221113184645-31828420/85/PVC-and-vinyl-polymers-pdf-1-320.jpg)

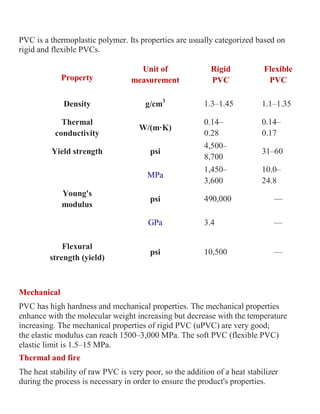

![The choice of additives used for the PVC finished product is controlled by the cost
performance requirements of the end use specification (underground pipe, window
frames, intravenous tubing and flooring all have very different ingredients to suit
their performance requirements).
Applications
PVC is used extensively in sewage pipes due to its low cost, chemical resistance
and ease of jointing
Pipes
Roughly half of the world's PVC resin manufactured annually is used for producing
pipes for municipal and industrial applications.[27]
In the private homeowner
market, it accounts for
66% 75% to of the household market
The use of chlorinated polyvinyl chloride (CPVC) pipe for use in residential
water supply piping systems.
Electric cables
PVC is commonly used as the insulation on electrical cables.
Flexible PVC coated wire and cable for electrical use has traditionally been
stabilised with lead, but these are being replaced with calcium-zinc based systems.
In a fire, PVC-coated wires can form hydrogen chloride fumes; the chlorine serves
to scavenge free radicals and is the source of the material's fire retardancy.
Hydrogen chloride fumes can also pose a health hazard. It dissolves in moisture and
breaks down onto surfaces, particularly in areas where the air is cool enough to
breathe, and is not available for inhalation.
Applications where smoke is a major hazard (notably in tunnels and communal
areas), PVC-free cable insulation is preferred, such as low smoke zero halogen
(LSZH) insulation.
Construction
If no plasticizers are added, it is known as uPVC (unplasticized polyvinyl chloride) or
rigid PVC.
uPVC is extensively used in the building industry as a low-maintenance material,](https://image.slidesharecdn.com/pvcandvinylpolymers-221113184645-31828420/85/PVC-and-vinyl-polymers-pdf-6-320.jpg)





![The glass transition temperature of polyvinyl acetate is between 30 and 45 °C depending on the
molecular weight.
PVAc emulsions such as Elmer's Glue-All contain polyvinyl alcohol as a protective colloid. In alkaline
conditions, boron compounds such as boric acid or borax cause the polyvinyl alcohol to cross-link,
forming tackifying precipitates or toys, such as Slime and Flubber.
A number of microorganisms can degrade polyvinyl acetate. Most commonly, damage is caused by
filamentous fungi—however algae, yeasts, lichens, and bacteria can also degrade polyvinyl acetate.[2]
Applications
As an emulsion in water, PVAc emulsions are used as adhesives for porous materials, particularly
for wood, paper, and cloth, and as a consolidant for porous building stone, in particular sandstone.[5]
Applications-
as wood glue, PVAc is known as "white glue" and the yellow as "carpenter's glue".
as paper adhesive during paper packaging conversion
in bookbinding and book arts, due to its flexible strong bond and non-acidic nature (unlike many
other polymers). The use of PVAc on the Archimedes Palimpsest during the 20th century greatly
hindered the task of disbinding the book and preserving and imaging the pages in the early 21st
century, in part because the glue was stronger than the parchment it held together.[6]
in handicrafts
as envelope adhesive
as wallpaper adhesive
as a primer for drywall and other substrates
as a gum base in chewing gum[7]
as an adhesive for cigarette paper[8]
The stiff homopolymer PVAc, but mostly the more soft copolymer, a combination of vinyl acetate and
ethylene, vinyl acetate ethylene (VAE), is also used in paper coatings, paint and other industrial
coatings, as a binder in nonwovens in glass fibers, sanitary napkins, filter paper and in textile
finishing.
Polyvinyl acetate is also the raw material to make other polymers like:
Polyvinyl alcohol -[HOCHCH2]-: Polyvinyl acetate is partially or completely hydrolysed to give
polyvinyl alcohol. This reversible saponification and esterification reaction was a strong hint
for Hermann Staudinger in the formulation of his theory of macromolecules.[9]
Polyvinyl acetate phthalate (PVAP): Polyvinyl acetate is partially hydrolyzed and then esterified
with phthalic acid.](https://image.slidesharecdn.com/pvcandvinylpolymers-221113184645-31828420/85/PVC-and-vinyl-polymers-pdf-12-320.jpg)