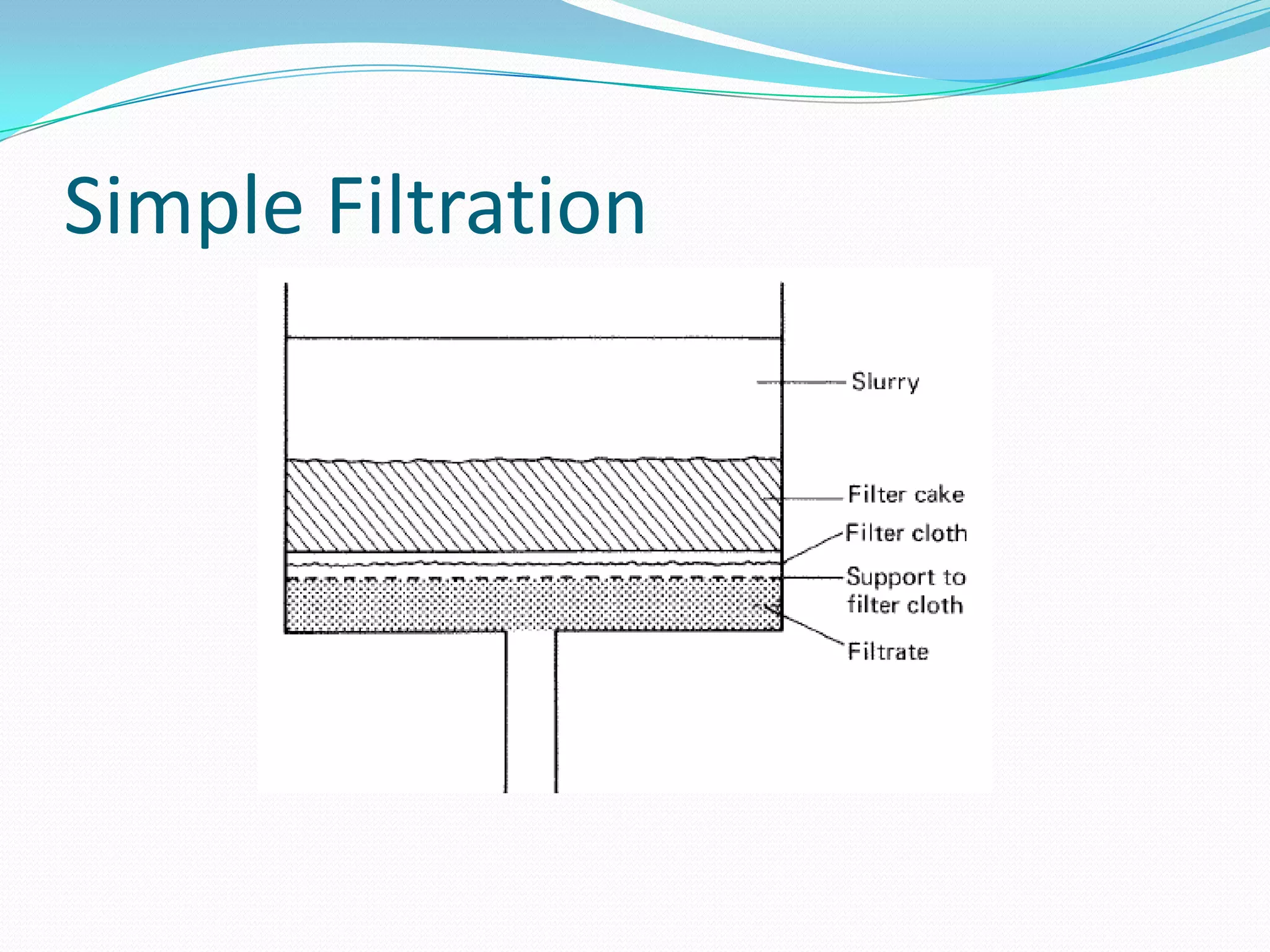
The document discusses various stages and techniques for recovering microbial products from fermentation broth. The first stage typically involves removing cells and debris through centrifugation or filtration. Next, the broth undergoes fractionation or extraction to separate components. Further purification may involve chromatography, crystallization, or membrane processes. The choice of recovery method depends on factors like the product's properties and stability. The goal is to obtain a highly purified product essentially free of impurities.