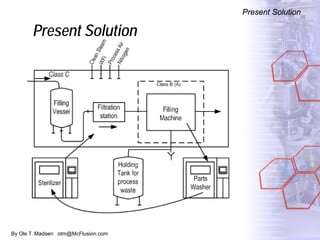
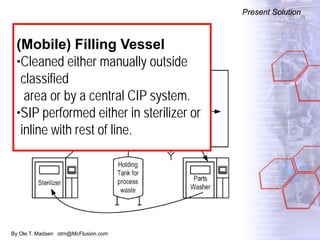
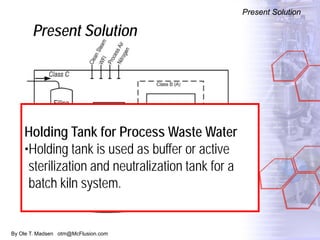
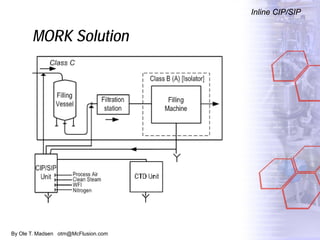
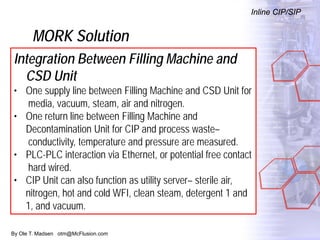
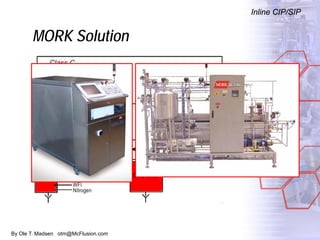
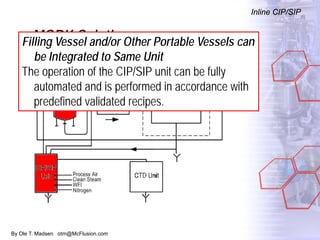
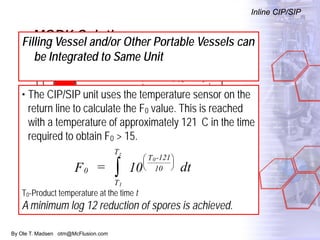
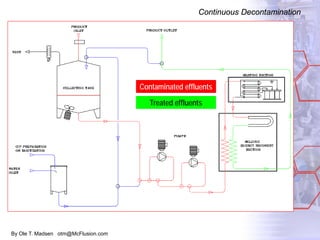
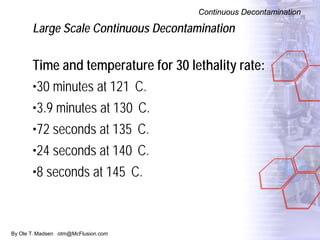
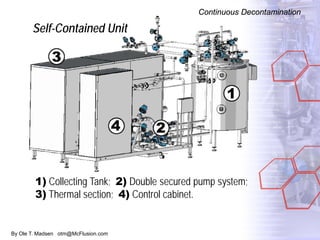
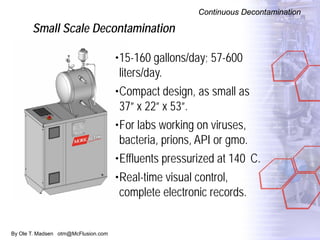
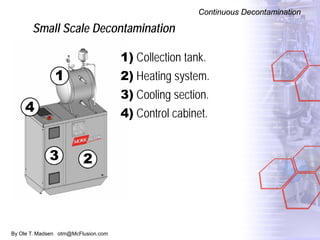
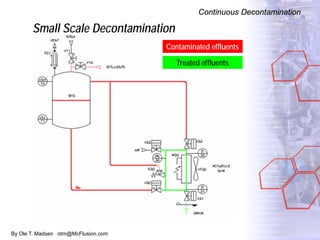
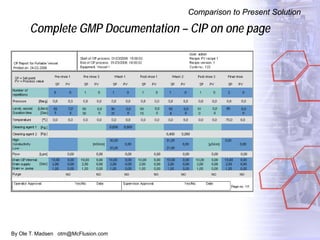
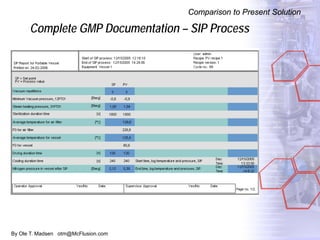
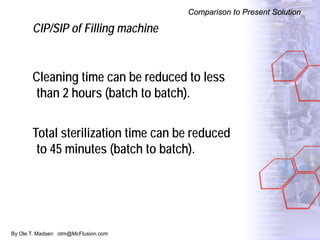
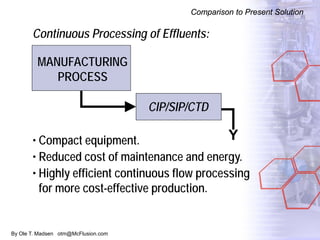
The document discusses new solutions for cleaning, sterilization, and decontamination of process equipment from McFlusion, including an inline CIP/SIP system for filling machines and continuous thermal decontamination of effluents. The inline CIP/SIP system would allow cleaning and sterilization to occur on the filling machine without disassembly, reducing batch changeover time. A continuous decontamination system is proposed to treat process waste water in a compact, efficient manner compared to batch systems. Advantages over current solutions include reduced cleaning time, lower costs, and ensuring waste meets regulations.