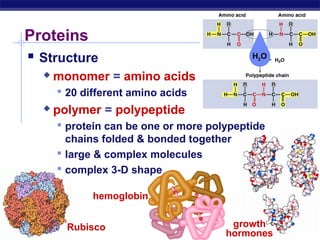
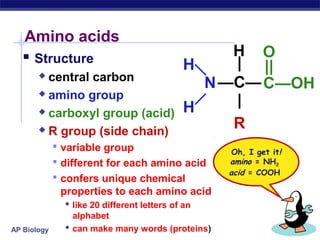
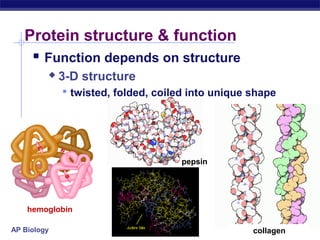
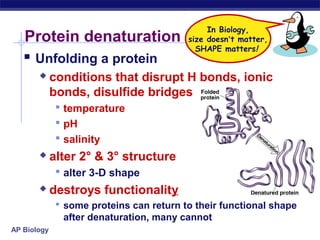
This document discusses proteins, which are complex biomolecules that perform a wide variety of essential functions in organisms. Proteins are made up of amino acid monomers that join together via peptide bonds to form polypeptide chains. The sequence and interactions of amino acid side chains determine a protein's unique 3D structure at the primary, secondary, tertiary, and sometimes quaternary levels. This complex structure is essential for a protein's specific functions, such as catalyzing reactions as enzymes, transporting molecules, or providing structure. Environmental factors can cause proteins to denature by disrupting bonds and changing shape, destroying their normal functions.