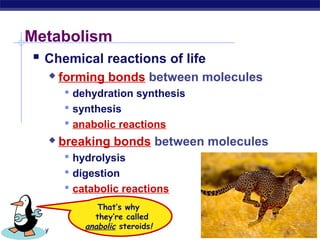
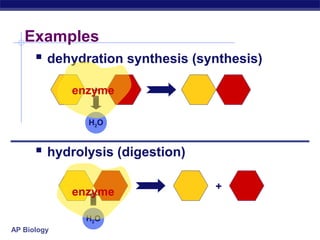
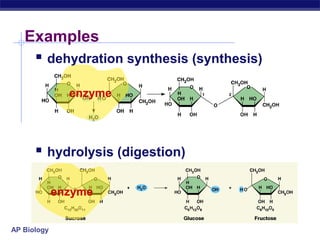
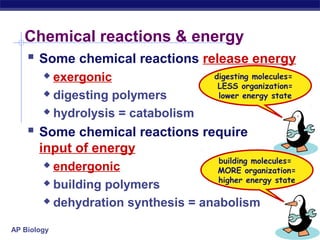
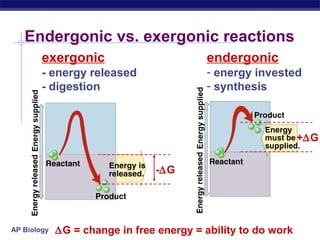
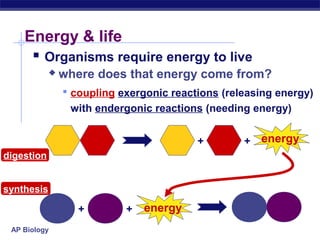
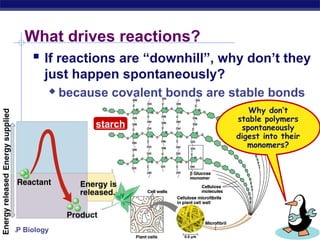
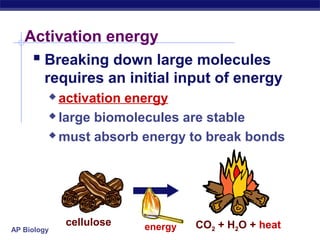
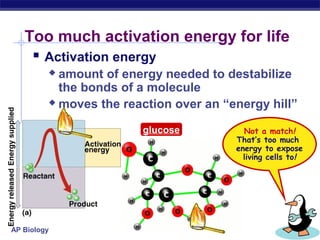
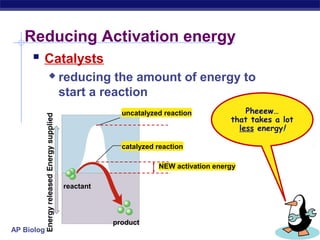
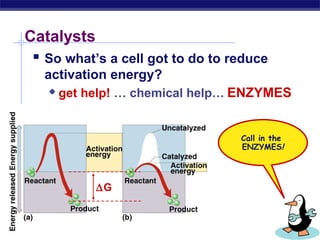
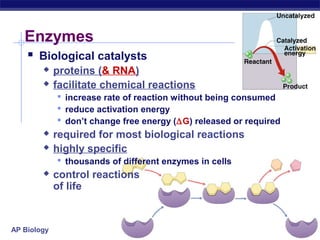
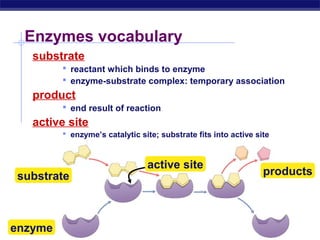
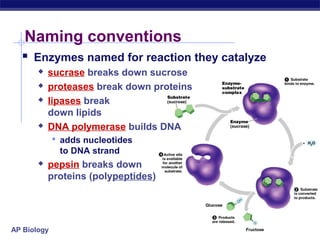
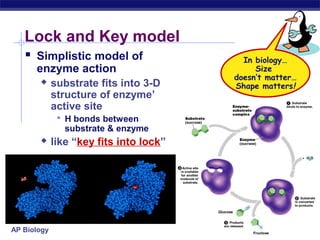
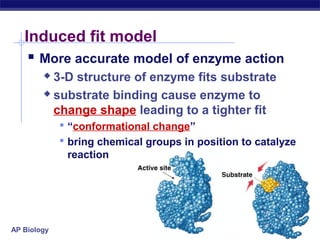
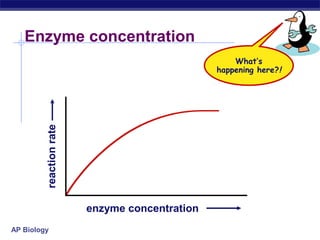
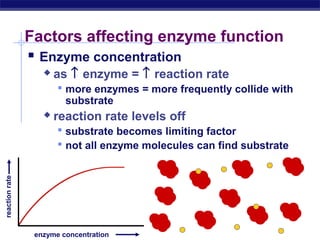
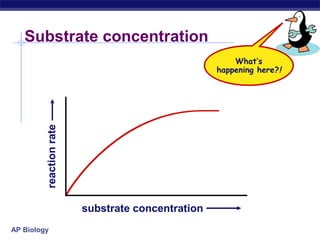
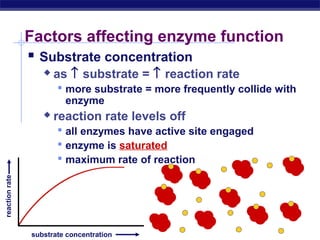
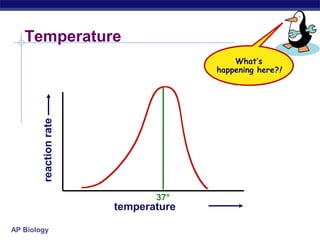
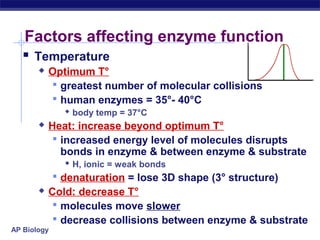
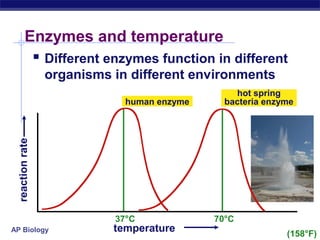
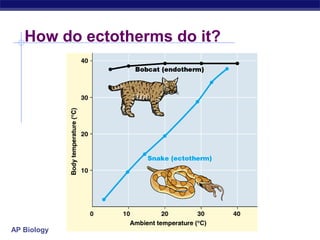
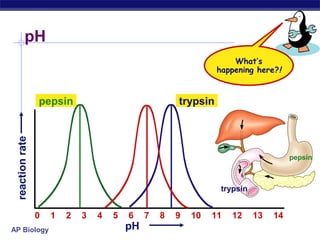
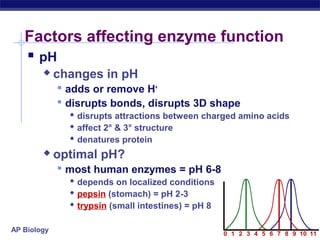
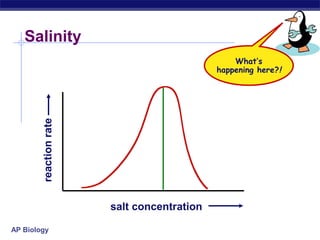
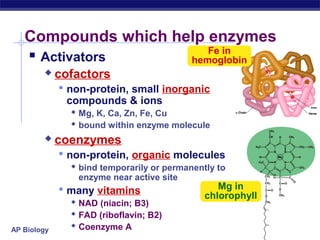
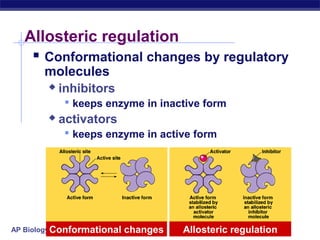
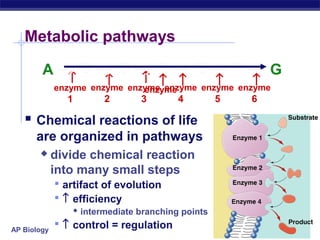
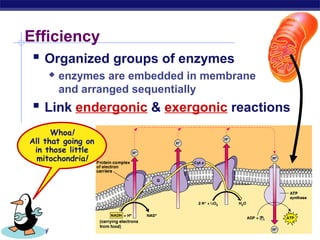
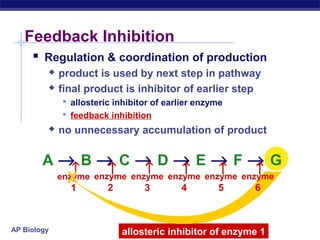
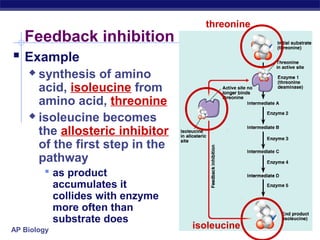
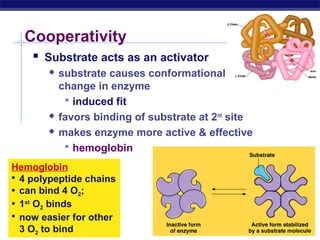
This document provides an overview of metabolism and enzymes for an AP Biology class. It discusses how chemical reactions in living things are facilitated by enzymes to transform energy and molecules. Enzymes are protein catalysts that reduce the activation energy of reactions and increase their rates. The functions of enzymes can be affected by factors like temperature, pH, and concentrations of enzymes and substrates. The document also explains inhibition of enzymes through competitive, noncompetitive, and irreversible mechanisms.