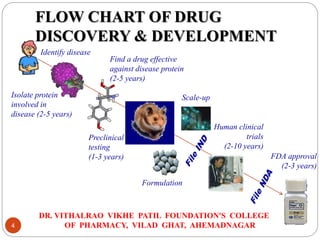
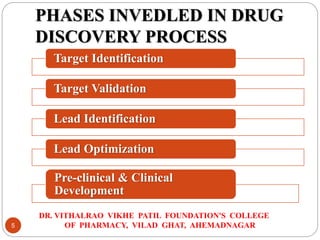
The document outlines the drug discovery process as taught at the Dr. Vithalrao Vikhe Patil Foundation's College of Pharmacy, detailing key phases including target identification, validation, lead identification, optimization, and preclinical and clinical development. It emphasizes the importance of various techniques such as high-throughput screening, combinatorial chemistry, and bioinformatics in identifying and developing effective drug candidates. The document also provides an overview of the clinical trial phases necessary for regulatory approval.






















![2)Combinatorial chemistry/synthesis
DR. VITHALRAO VIKHE PATIL FOUNDATION'S COLLEGE
OF PHARMACY, VILAD GHAT, AHEMADNAGAR
28
“ The automated synthesis of a large number of compounds in
a short time period using a defined reaction route and a
large variety of reactants, Normally carried out on small
scale using solid phase synthesis and automated synthetic
machines”
Tech used in combinatorial chemistry
A. Solid phase tech.
1] Solid phase synthesis
2] Parallel synthesis
a) Hoghton’s tea bag procedure
b) Automated parallel synthesis](https://image.slidesharecdn.com/drugdiscoveryprocess-210303132127/85/Drug-discovery-process-28-320.jpg)
![Conti…
DR. VITHALRAO VIKHE PATIL FOUNDATION'S COLLEGE
OF PHARMACY, VILAD GHAT, AHEMADNAGAR
29
3] Mixed combinatorial synthesis
4] Mixed & split synthesis
Combinatorial chemistry plays a central role because of its ability to
create and produce molecules. In addition, combinatorial chemistry is
an active participant in drug development from the synthesis of
substances until its bulk production. Combinatorial chemistry can
create large population of analogs of a given scaffold.
The analogs are synthesize in successive steps with the use of
robotics. The analogs are included in “combinatorial libraries”
consisting of a great number of wells, each one containing in
solution several dozens of compounds.
Only a few milligrams of compounds can be obtained by this
method, however this is generally sufficient for primary
biological screening.](https://image.slidesharecdn.com/drugdiscoveryprocess-210303132127/85/Drug-discovery-process-29-320.jpg)





![Phases of clinical trials
DR. VITHALRAO VIKHE PATIL FOUNDATION'S COLLEGE
OF PHARMACY, VILAD GHAT, AHEMADNAGAR
35
Phase 1 - A small group of healthy volunteers (20-100) are
selected to assess the safety, tolerability, pharmacokinetics,
& pharmacodynamics of a therapy. Normally include dose
ranging studies so that doses for clinical use can be
set/adjusted.[6 months-1 yr]
Phase 2- Performed on larger groups (100-200) & are
designed to assess the activity of the therapy and
effectiveness of drug & continue phase1 safety
assessments.[2-3 yrs]](https://image.slidesharecdn.com/drugdiscoveryprocess-210303132127/85/Drug-discovery-process-35-320.jpg)
![Conti..
DR. VITHALRAO VIKHE PATIL FOUNDATION'S COLLEGE
OF PHARMACY, VILAD GHAT, AHEMADNAGAR
36
Phase 3 -Randomized controlled trials on large patient groups
[1000-5000] [5yr]
assessment of the efficacy of the new therapy,
side effects are also monitored.
to obtain approval from the FDA This document is
submitted to the FDA for review.
Phase 4 –
After regulatory authority approval
Larger no. of patients.
DDI and dose response studies & safety studies](https://image.slidesharecdn.com/drugdiscoveryprocess-210303132127/85/Drug-discovery-process-36-320.jpg)



