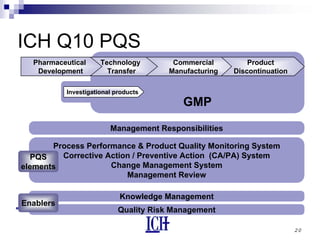
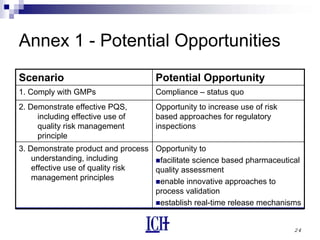
The document summarizes the ICH Q10 guideline, which provides a framework for pharmaceutical quality systems throughout the lifecycle of pharmaceutical products. The guideline aims to facilitate continual improvement and quality risk management. It augments good manufacturing practices and covers topics like management responsibility, process performance monitoring, change management, and knowledge management. When combined with ICH Q8 on pharmaceutical development and ICH Q9 on quality risk management, ICH Q10 provides a harmonized approach to ensuring product quality.











![12
Content: §3.1 Lifecycle Stage Goals
1 Pharmaceutical Development
design product and process to consistently deliver the
intended performance and meet the needs of [parties]
exploratory and clinical development studies are inputs
2 Technology Transfer
transfer product/process knowledge between
development and manufacturing, and within or between
sites](https://image.slidesharecdn.com/q10generalpresentation-160119073738/85/Q10-general-presentation-12-320.jpg)