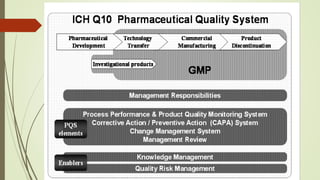
This document provides an overview of ICH Q10, which describes a pharmaceutical quality system model based on ISO quality concepts and GMP regulations. The quality system can be implemented throughout a product's lifecycle to facilitate innovation, continual improvement, and strengthen the link between development and manufacturing. ICH Q10 augments GMPs by describing specific quality system elements and management responsibilities to achieve product realization, establish state of control, and facilitate continual improvement.