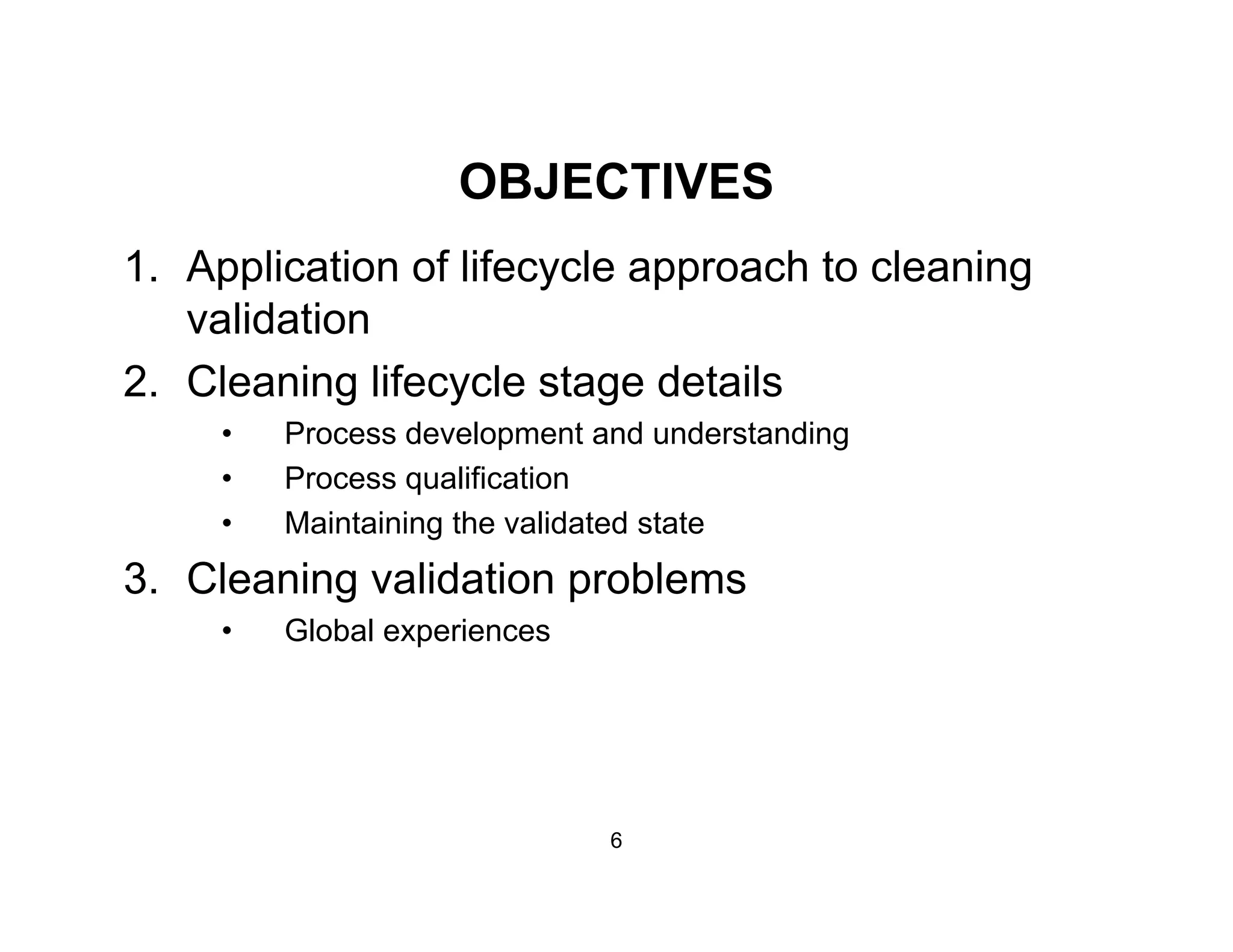
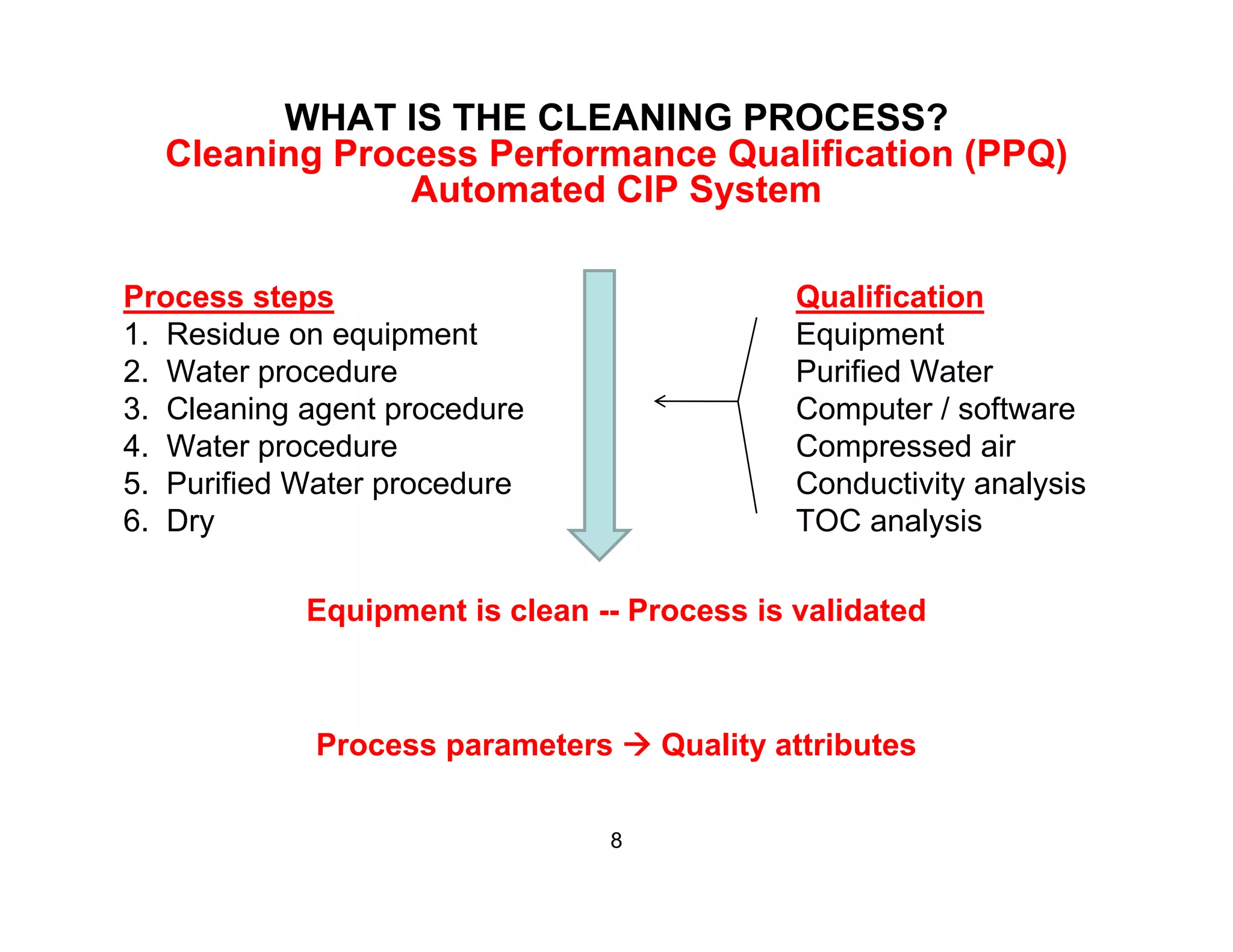
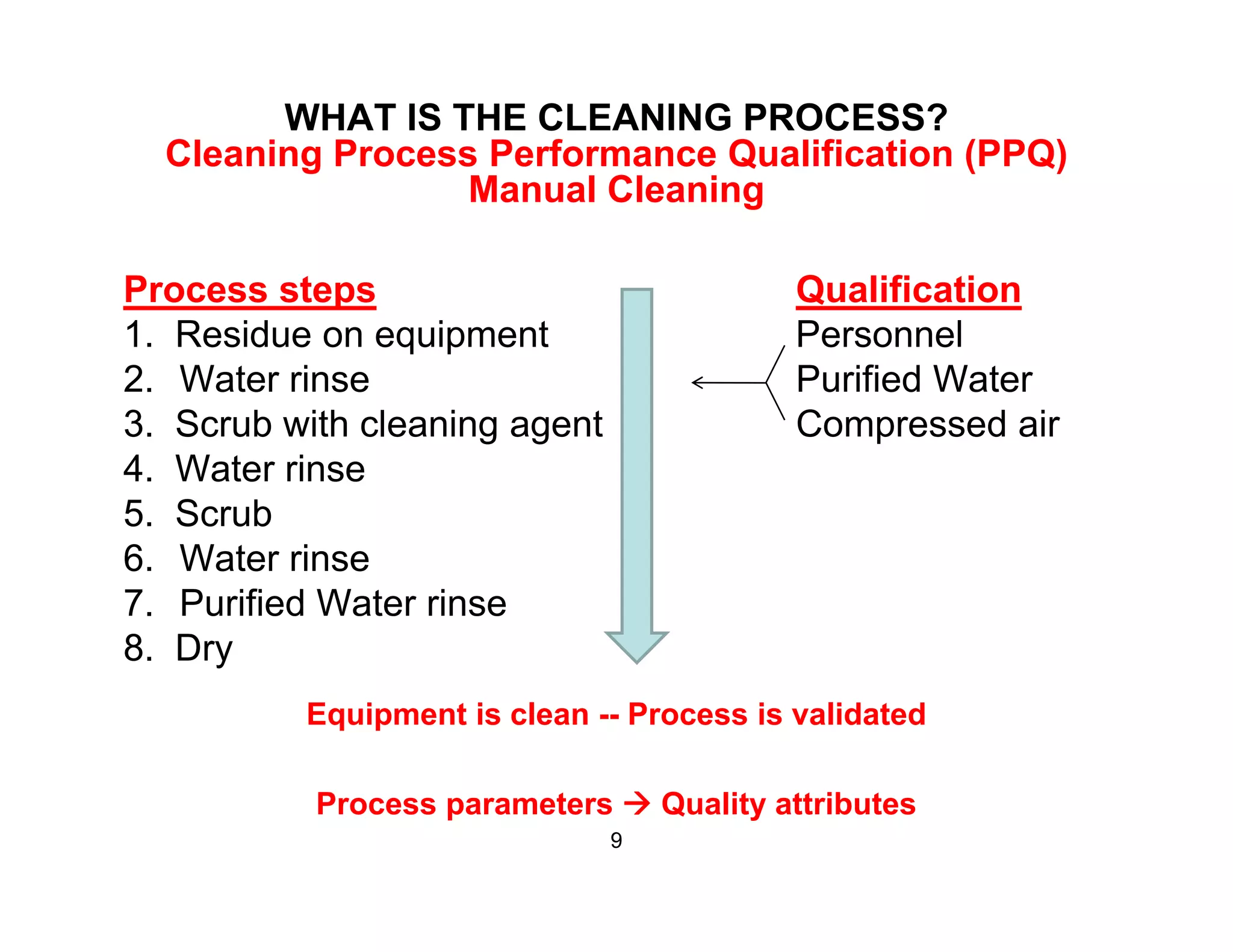
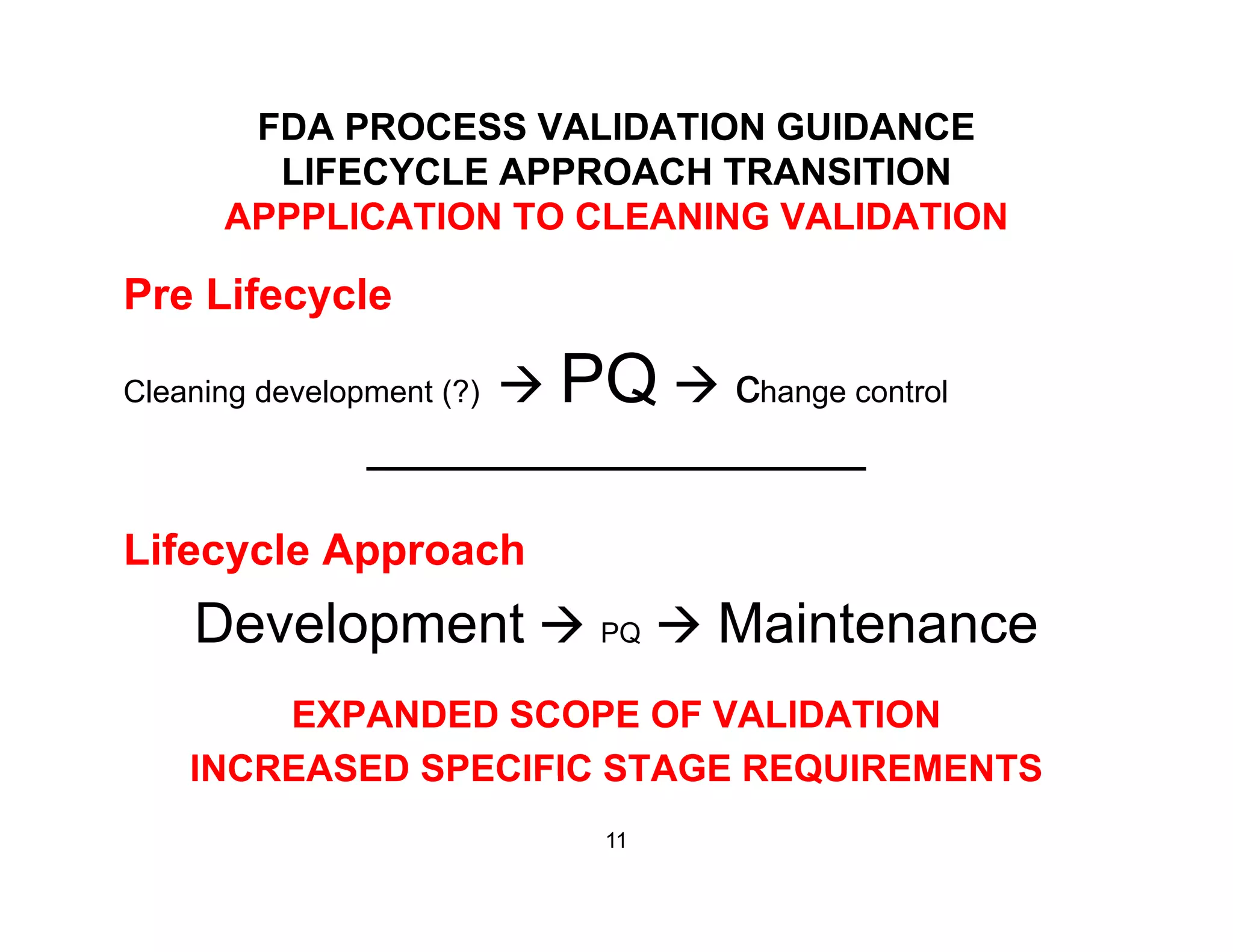
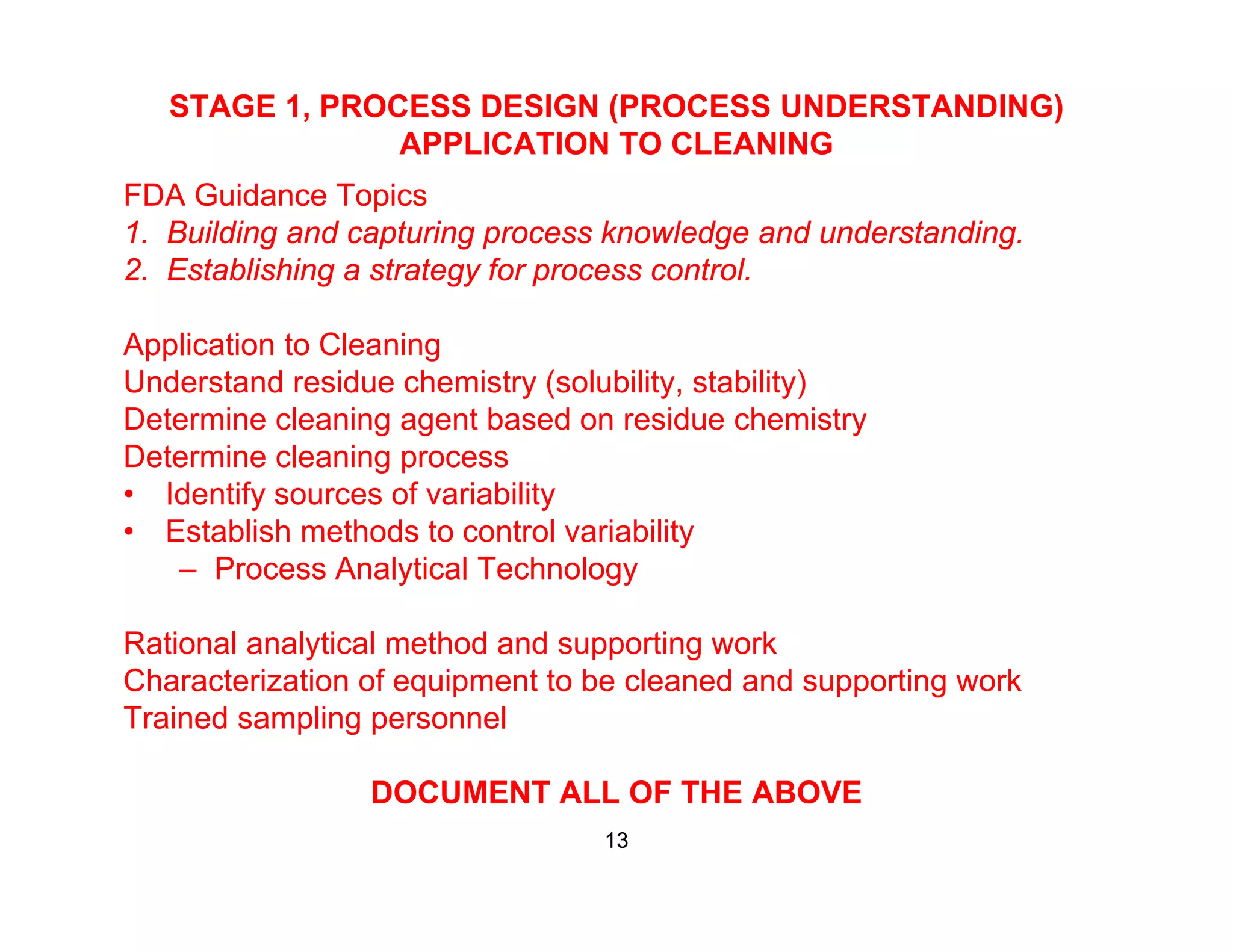
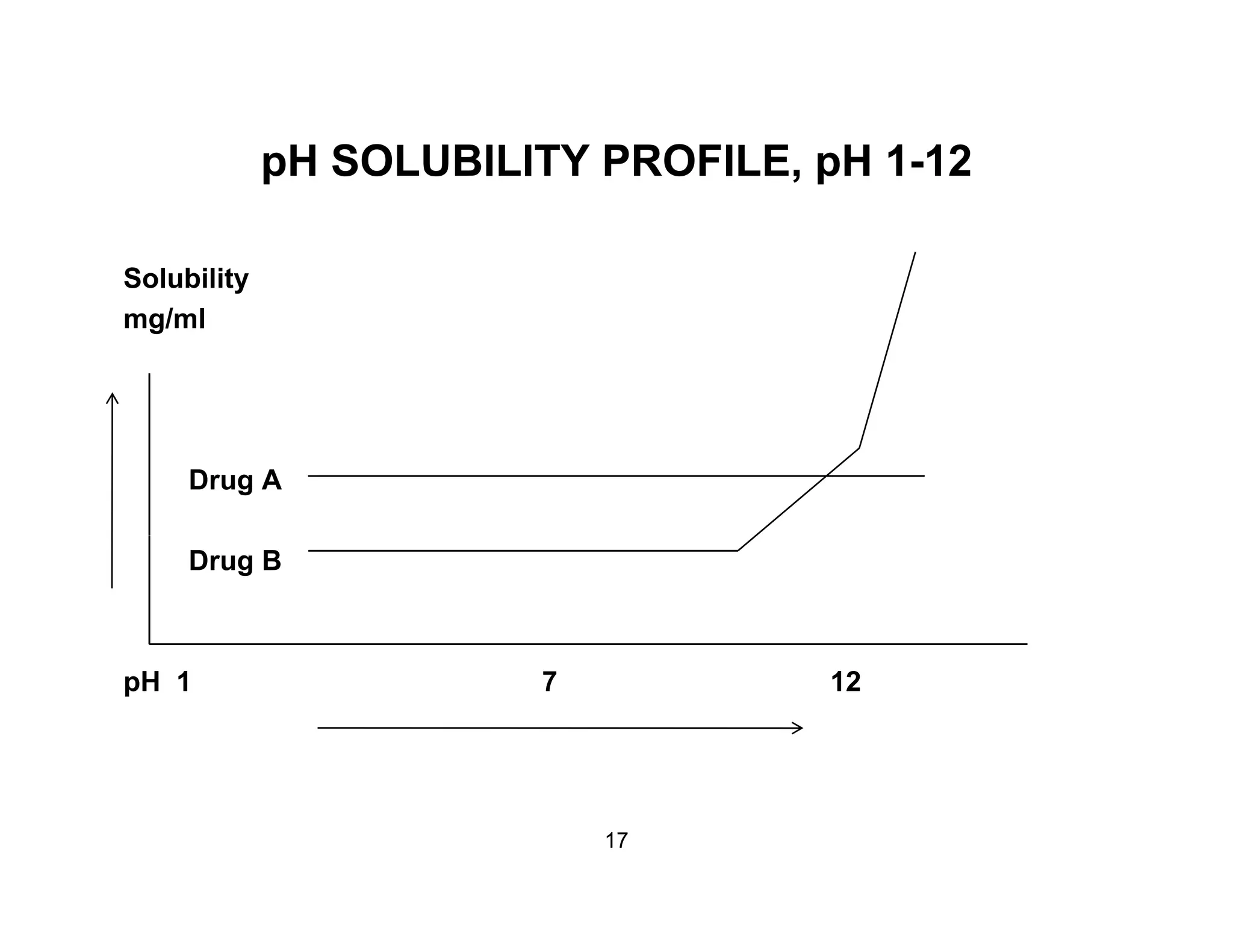
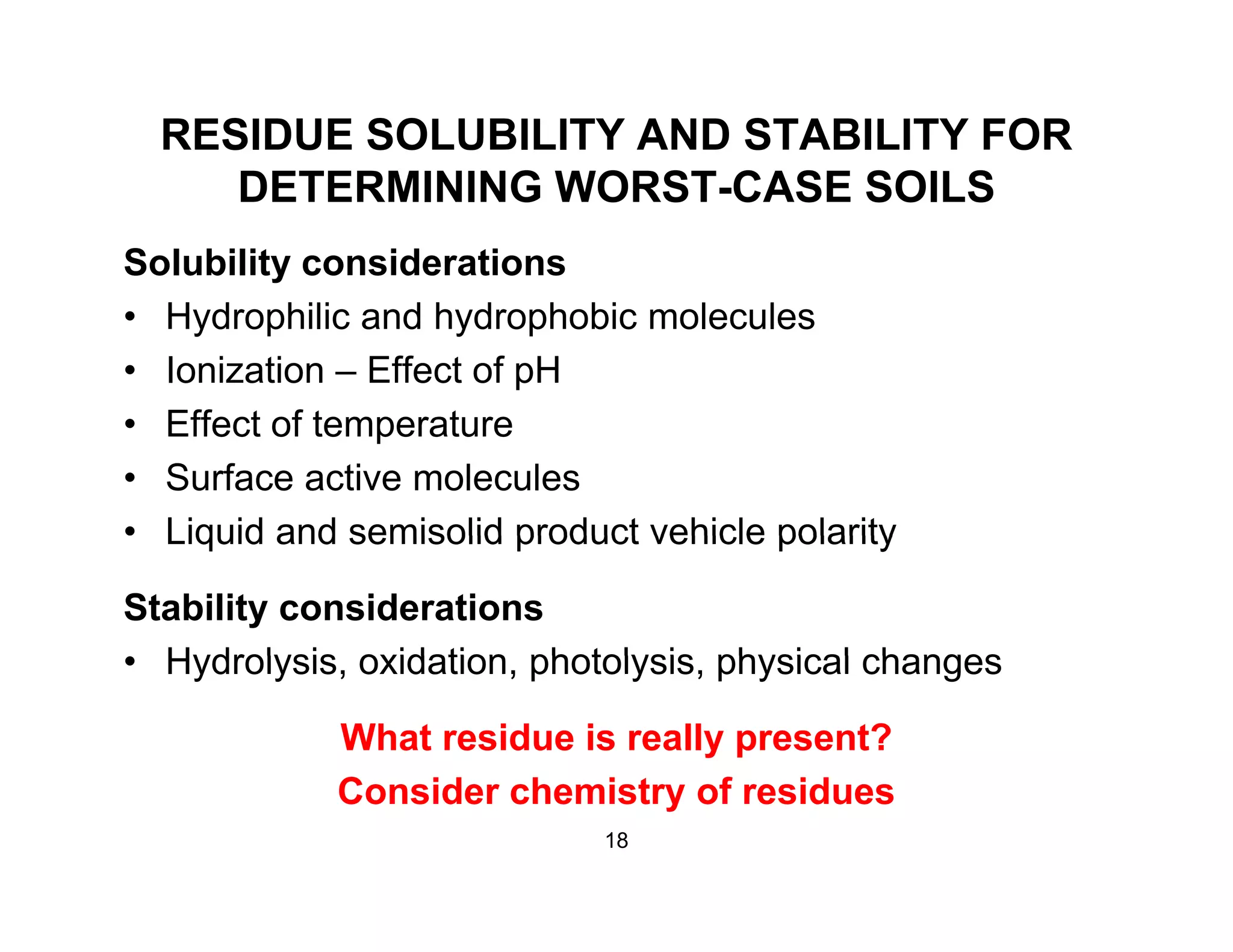
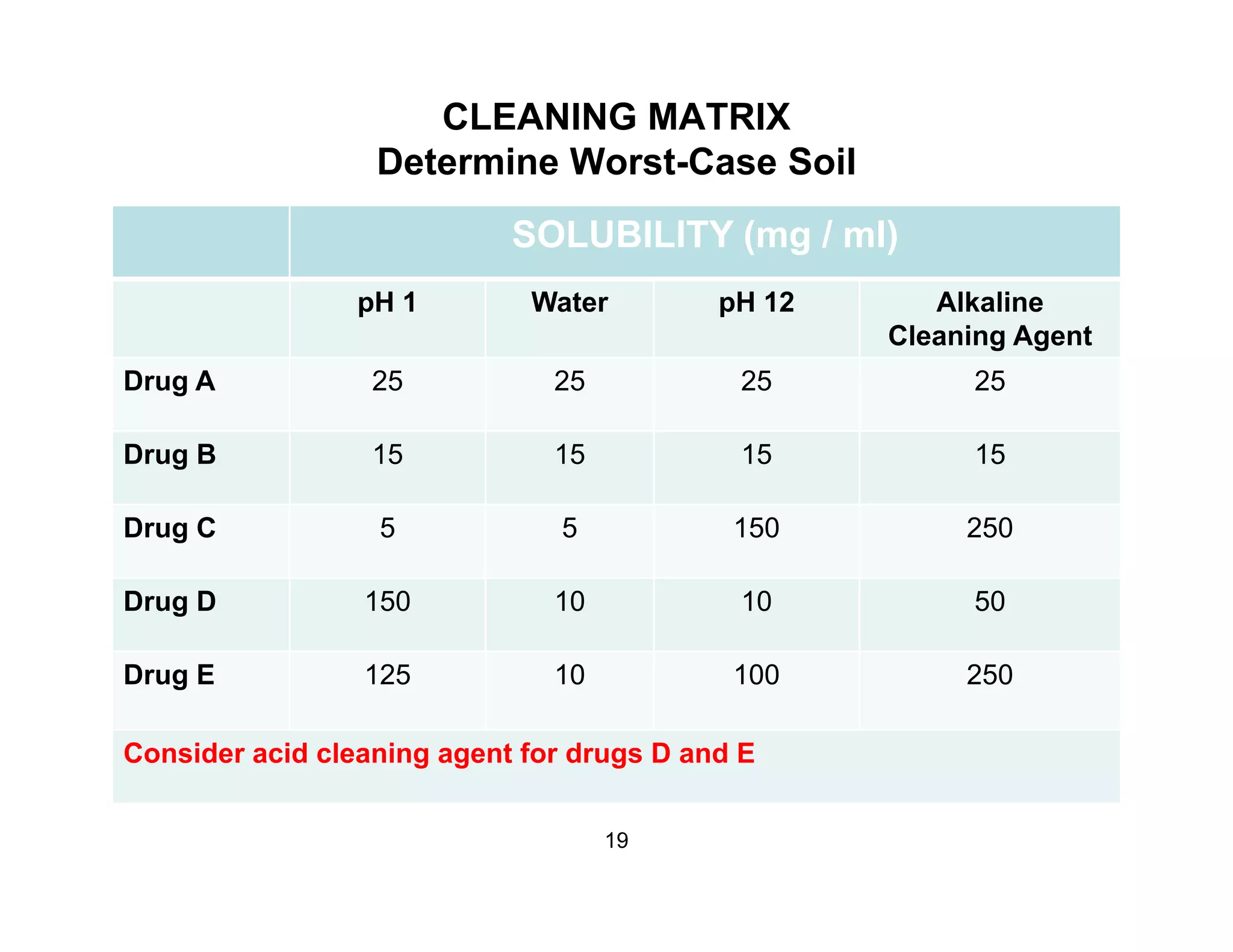
The document discusses a lifecycle approach to cleaning validation, emphasizing the importance of training and adherence to procedures for effective cleaning practices in manufacturing. It outlines stages of the cleaning validation process, highlighting the necessity of understanding residue chemistry, analytical methods, and the implications of manual versus automated cleaning methods. The document stresses the need for consistent documentation and monitoring to ensure compliance and efficiency in cleaning validation efforts.