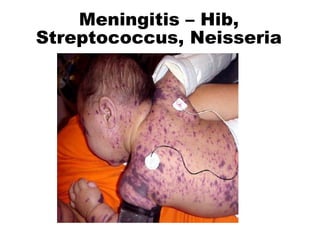
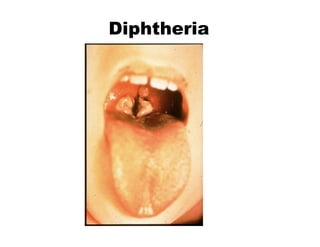
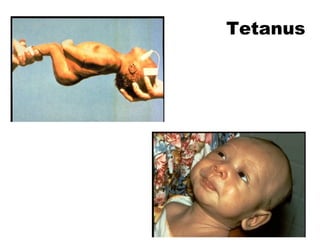
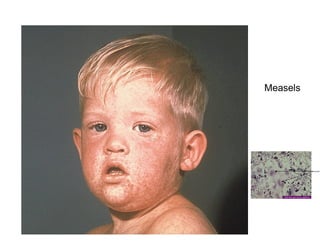
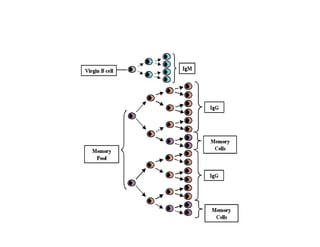
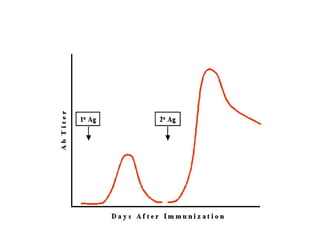
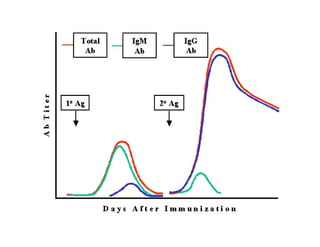
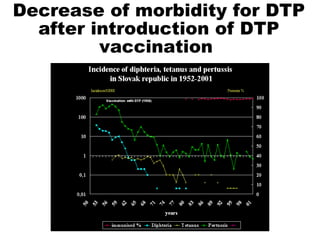
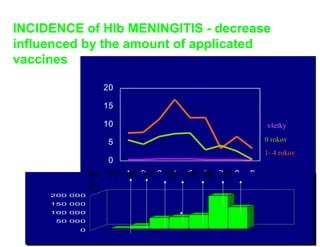
This document discusses the principles of vaccination. It covers:
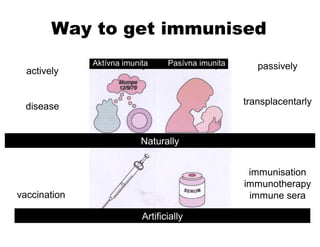
- Active and passive immunity and how they are obtained naturally or artificially through vaccination or antibody transfer.
- The components of vaccines, including antigens, antibodies, and epitopes.
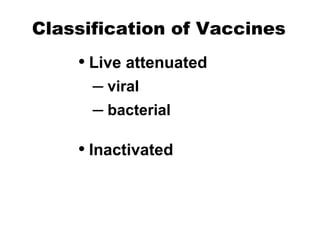
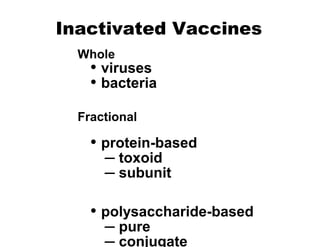
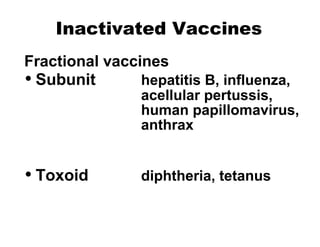
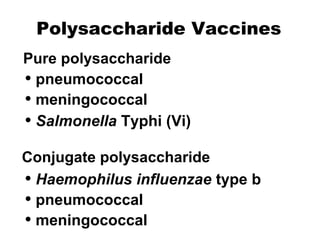
- The different types of vaccines such as live attenuated, inactivated, toxoid, subunit, and polysaccharide vaccines.
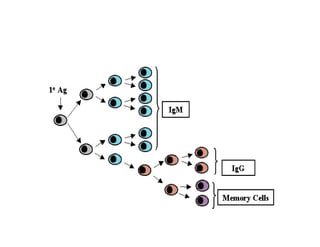
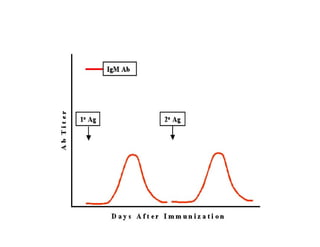
- How vaccines work to produce immunity through replication or exposure to antigens without causing disease.
- The development process, safety testing, and surveillance of new vaccines.