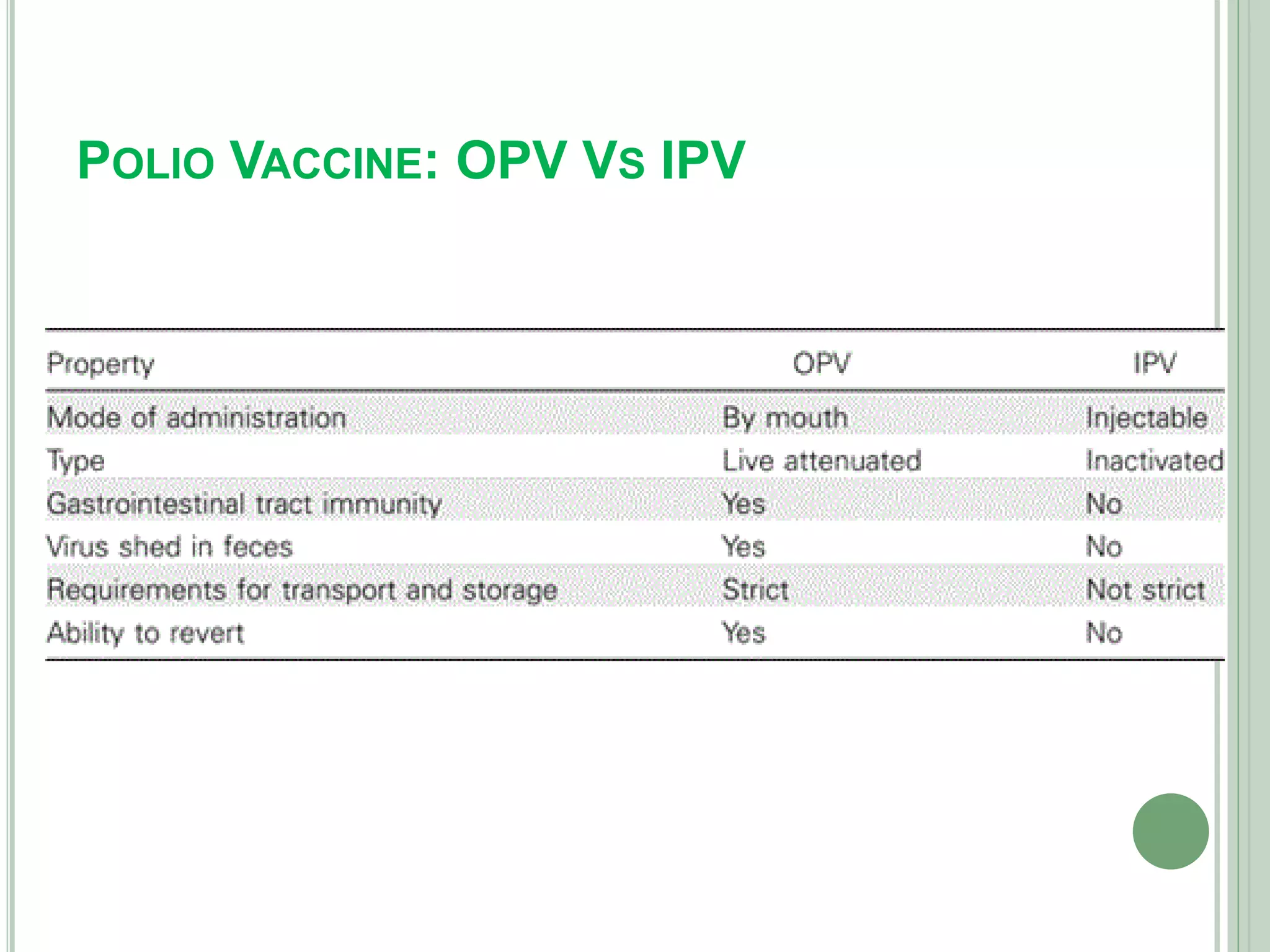
The document discusses vaccines, defining them as immunobiological substances designed to provide specific disease protection, and outlines various types including live attenuated, inactivated, toxoid, and DNA vaccines. It highlights the importance of the Expanded Program on Immunization (EPI) in Nepal, particularly regarding Oral Polio Vaccine (OPV) and Inactivated Polio Vaccine (IPV), and presents rationale for transitioning from OPV to IPV to mitigate risks of vaccine-derived poliovirus. Additionally, it discusses the benefits and risks associated with each vaccine type and their respective immunization strategies.