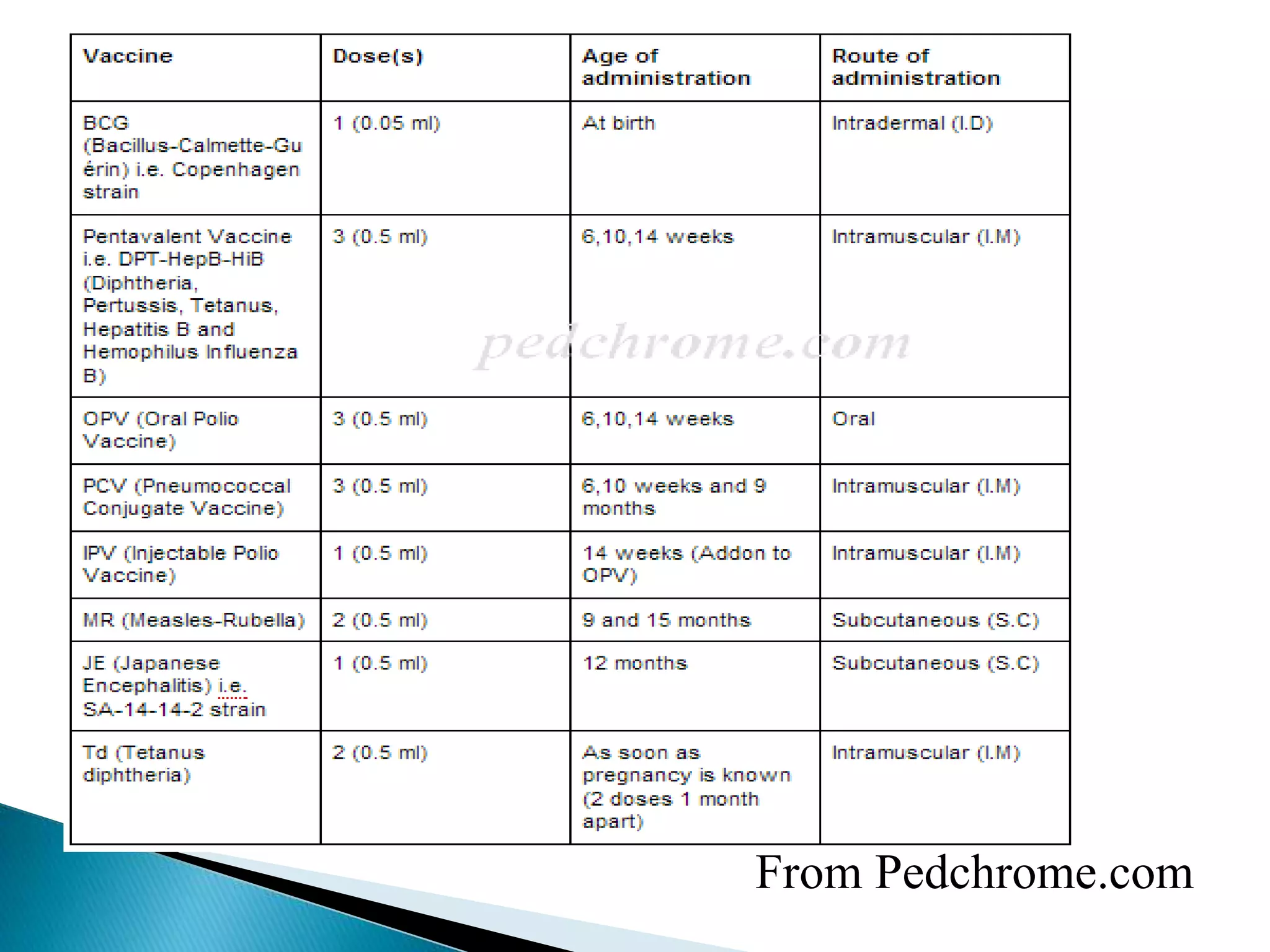
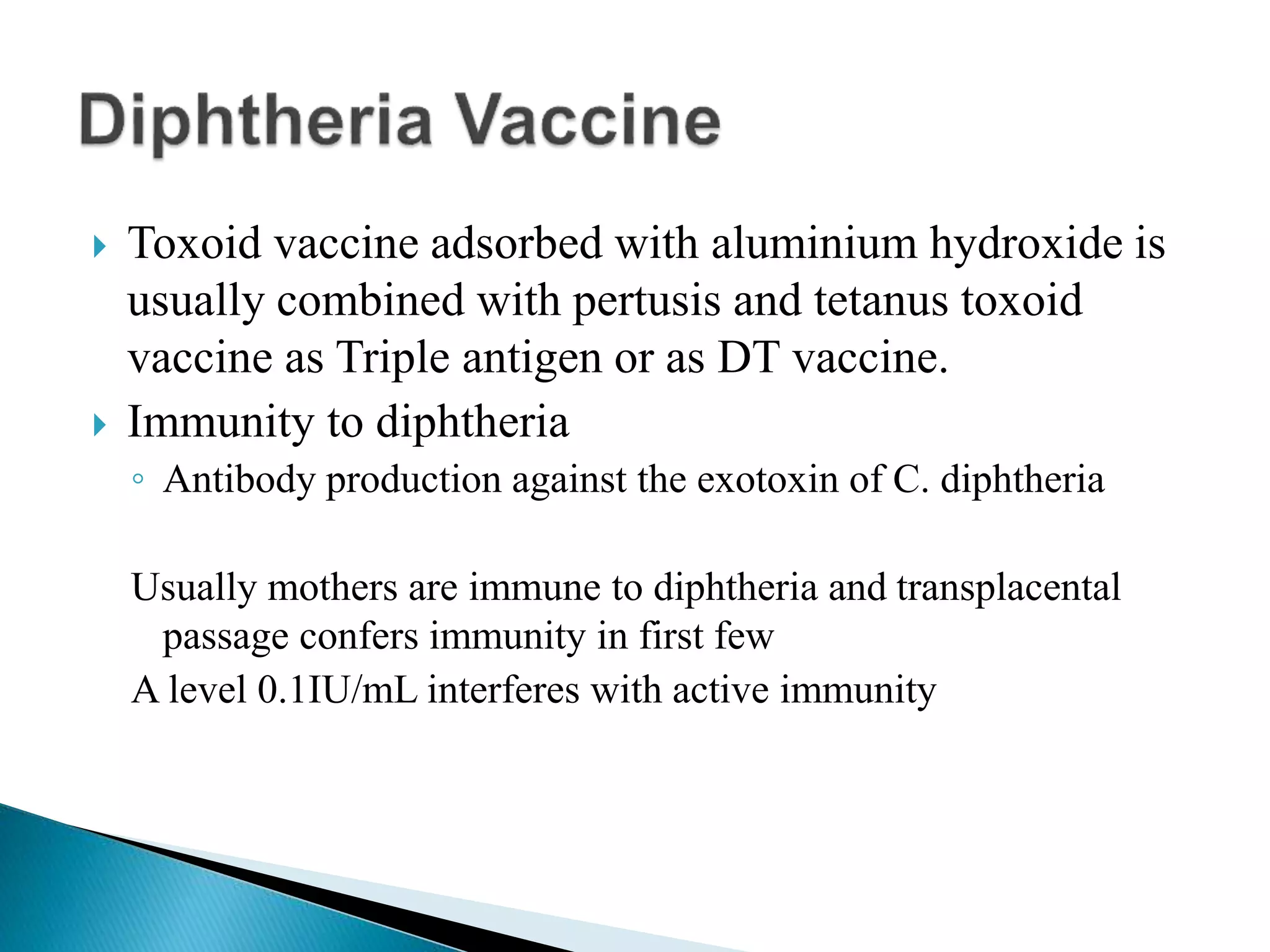
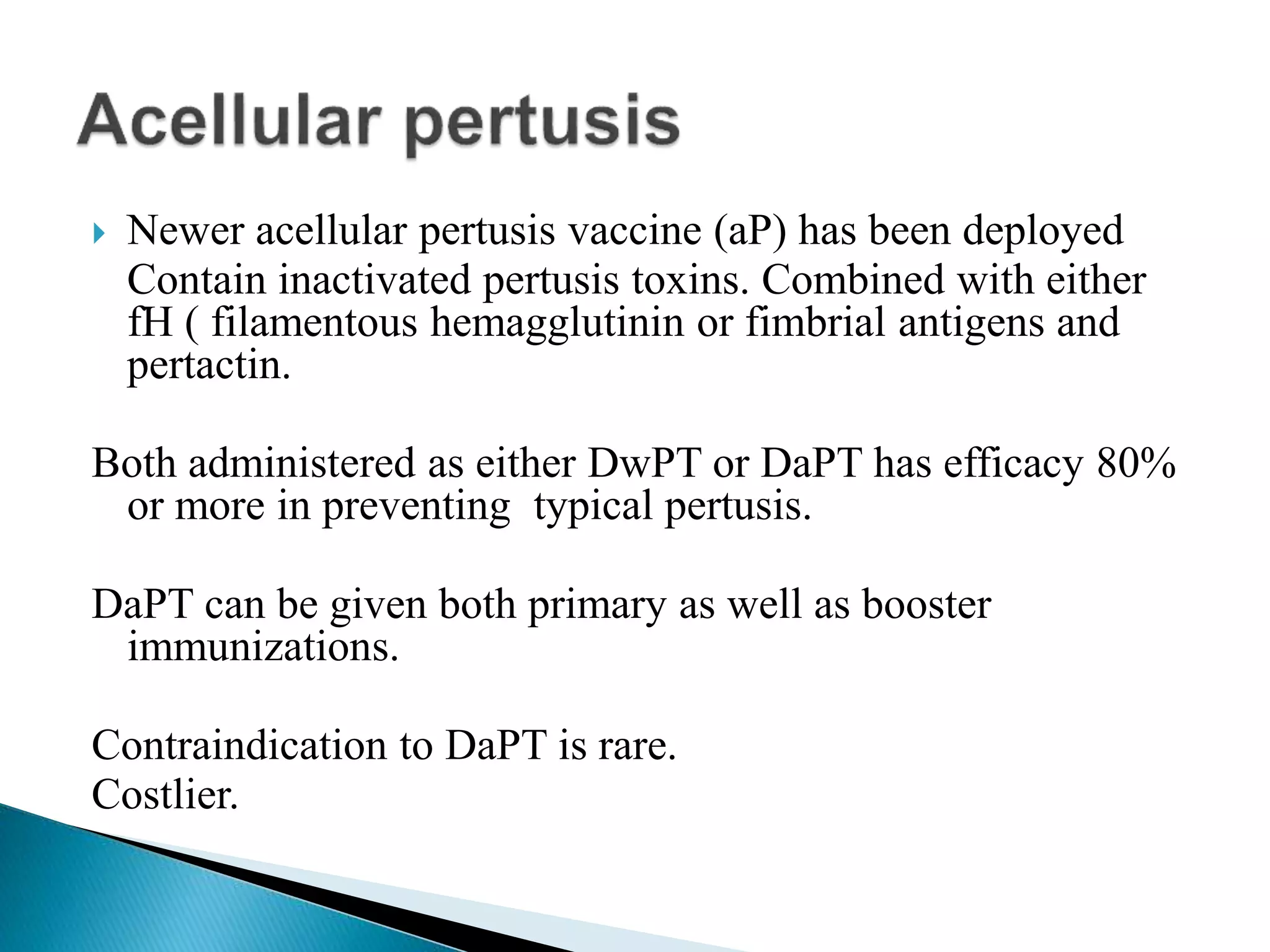
This document discusses immunization and different types of vaccines. It describes passive and active immunization. Passive immunization provides immediate short-term protection from antibodies without immune system activation, while active immunization activates the immune system to produce long-lasting immunity. The document outlines various vaccine types including live attenuated, inactivated, toxoid, and subunit vaccines. It provides details on vaccine administration, schedules, and contraindications.