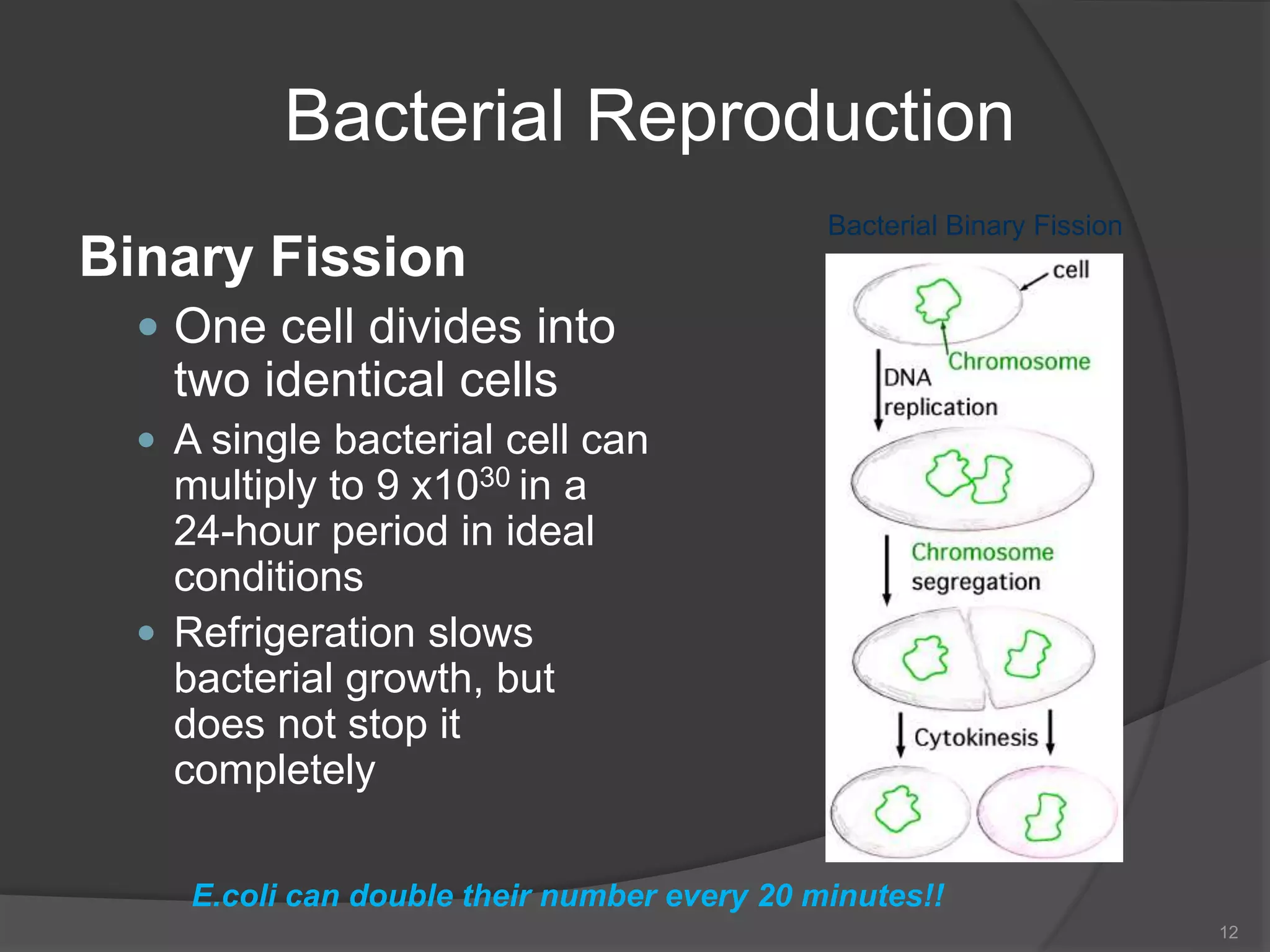
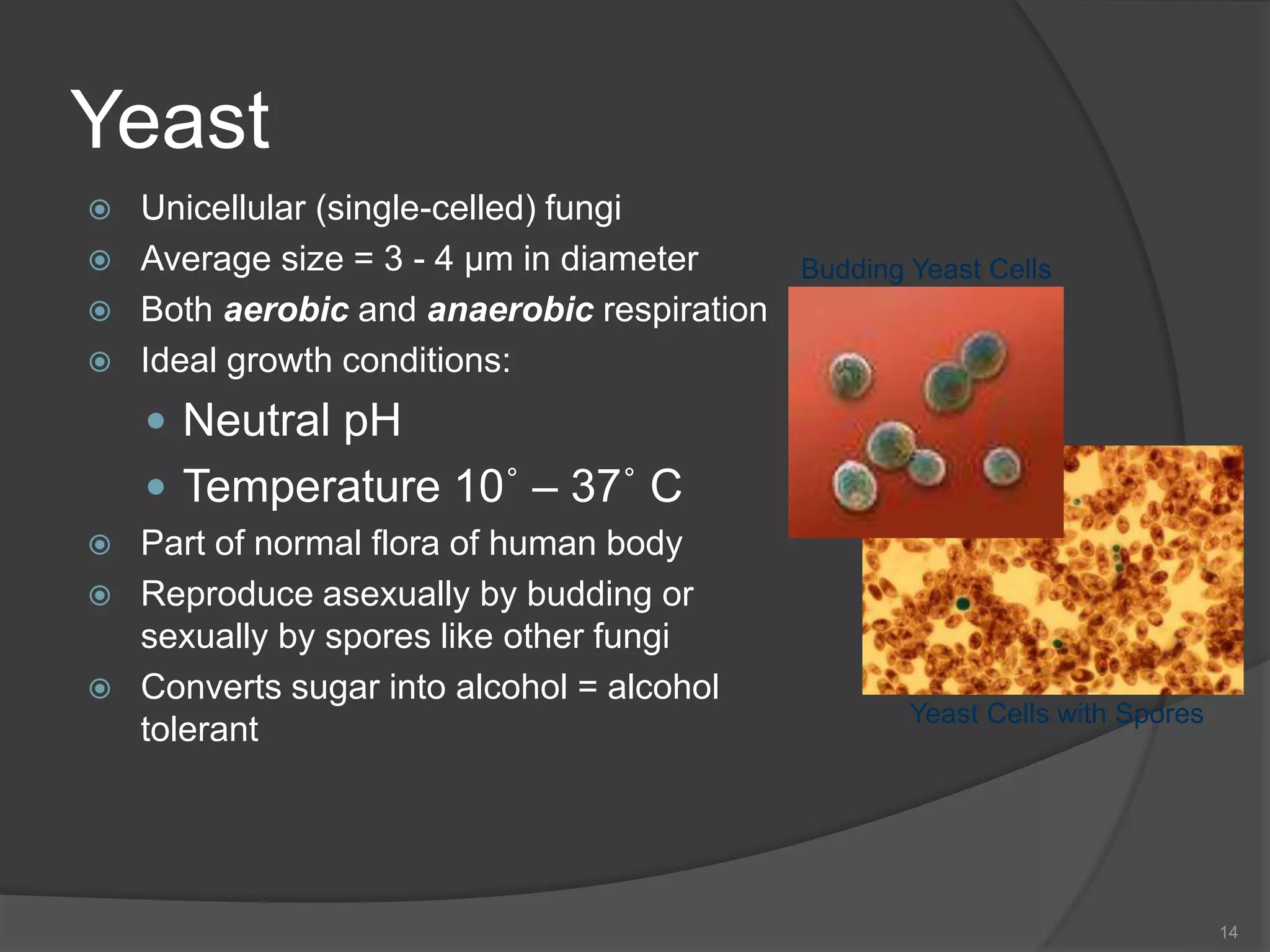
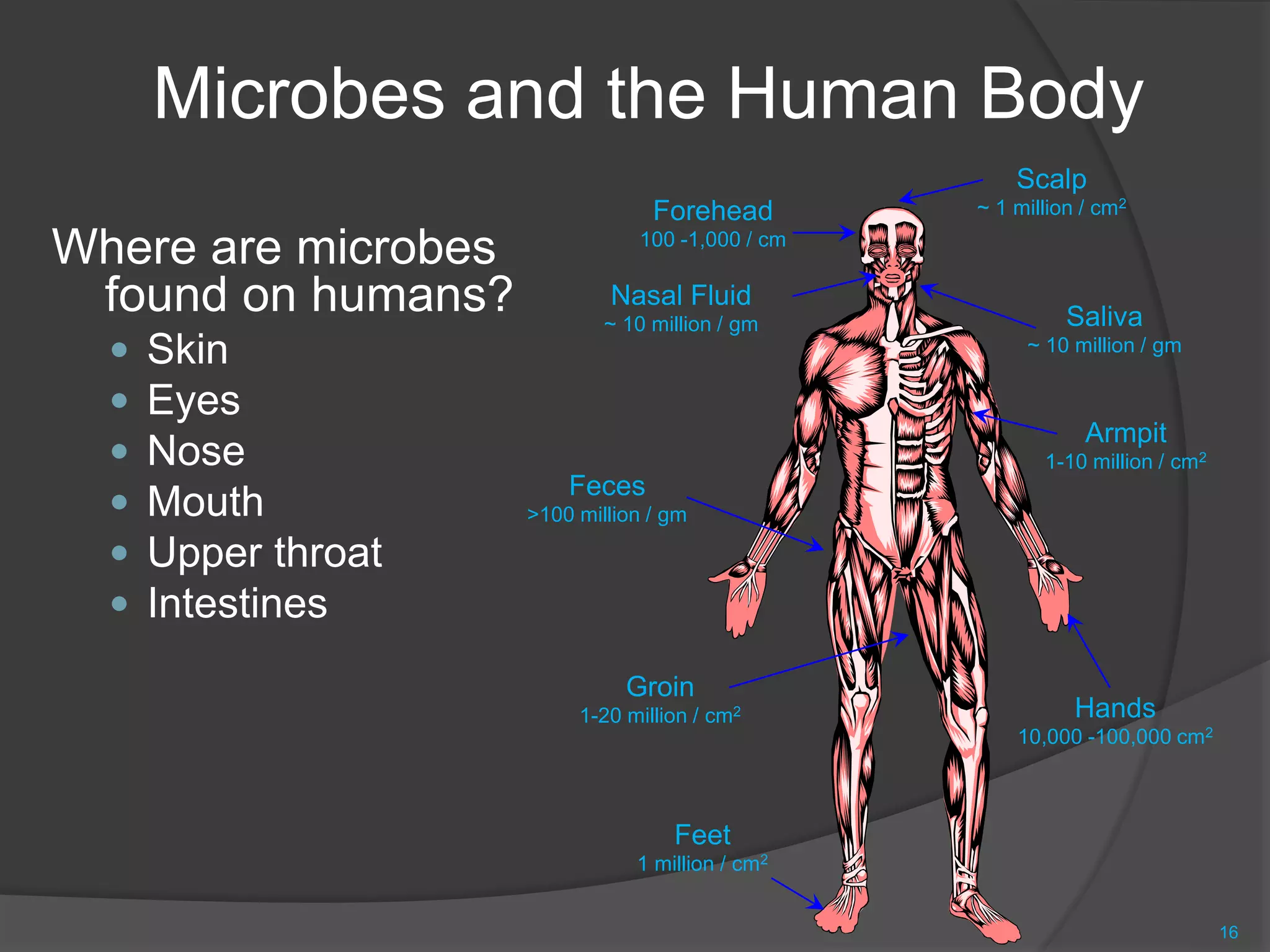
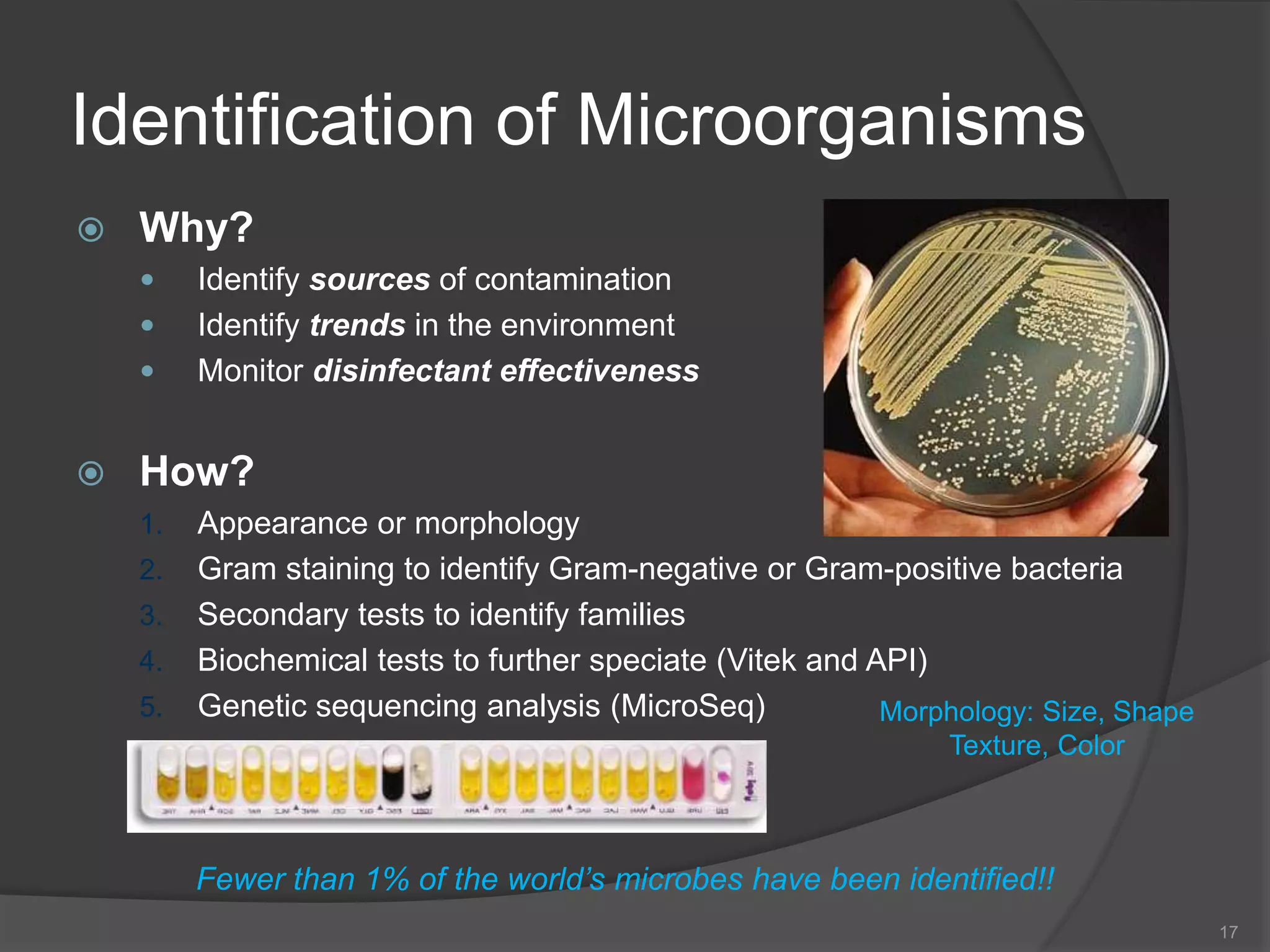
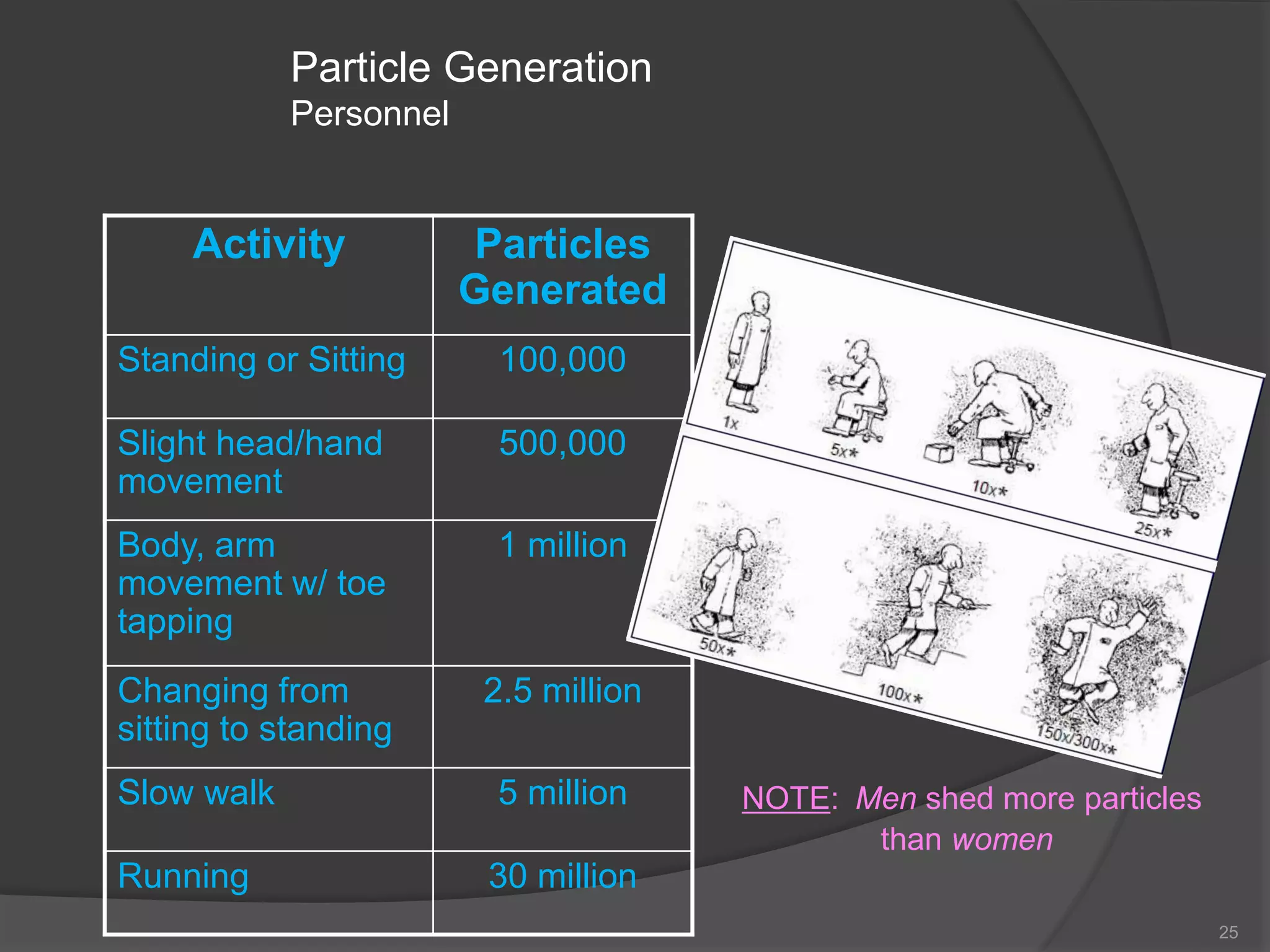
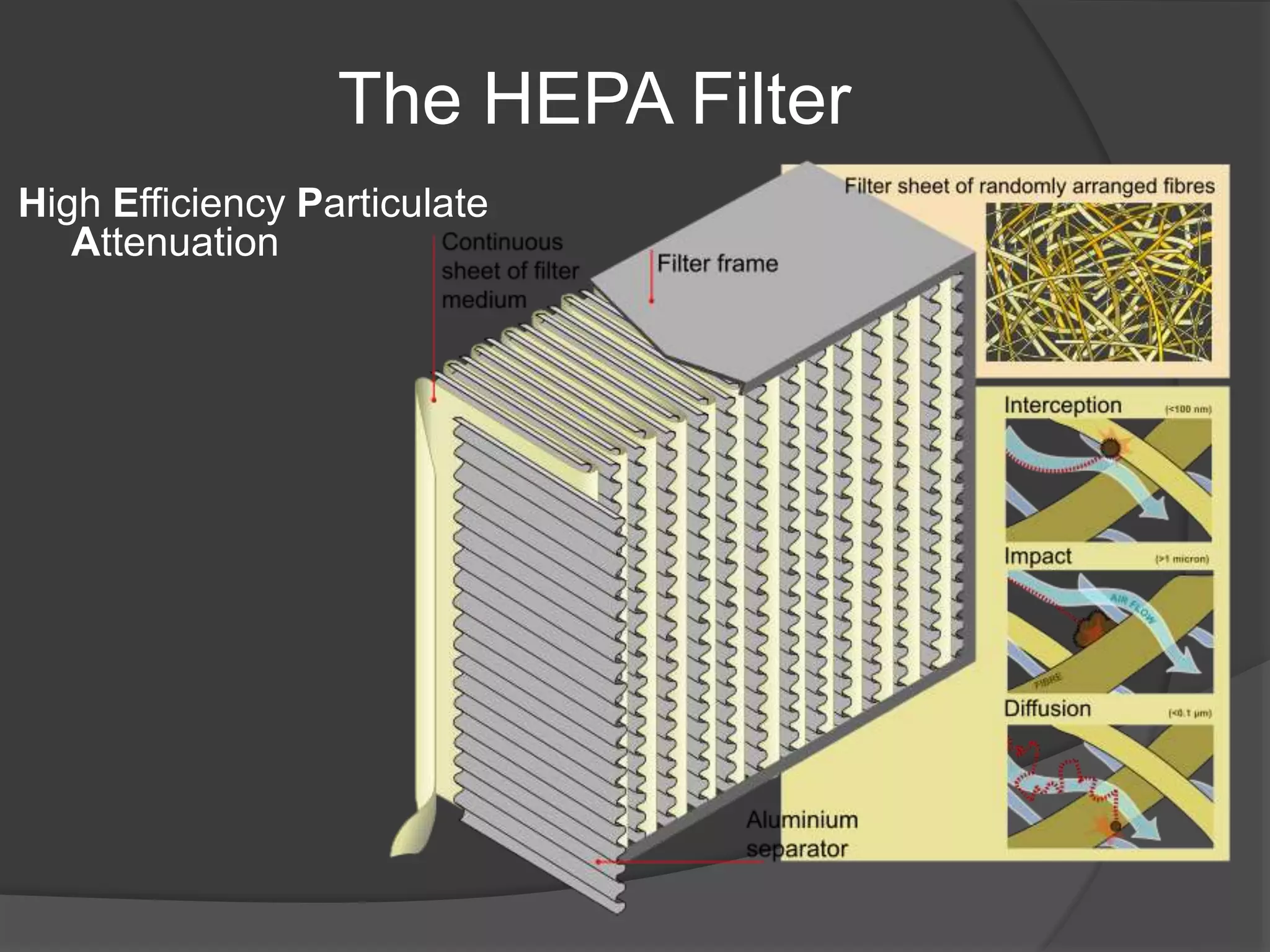
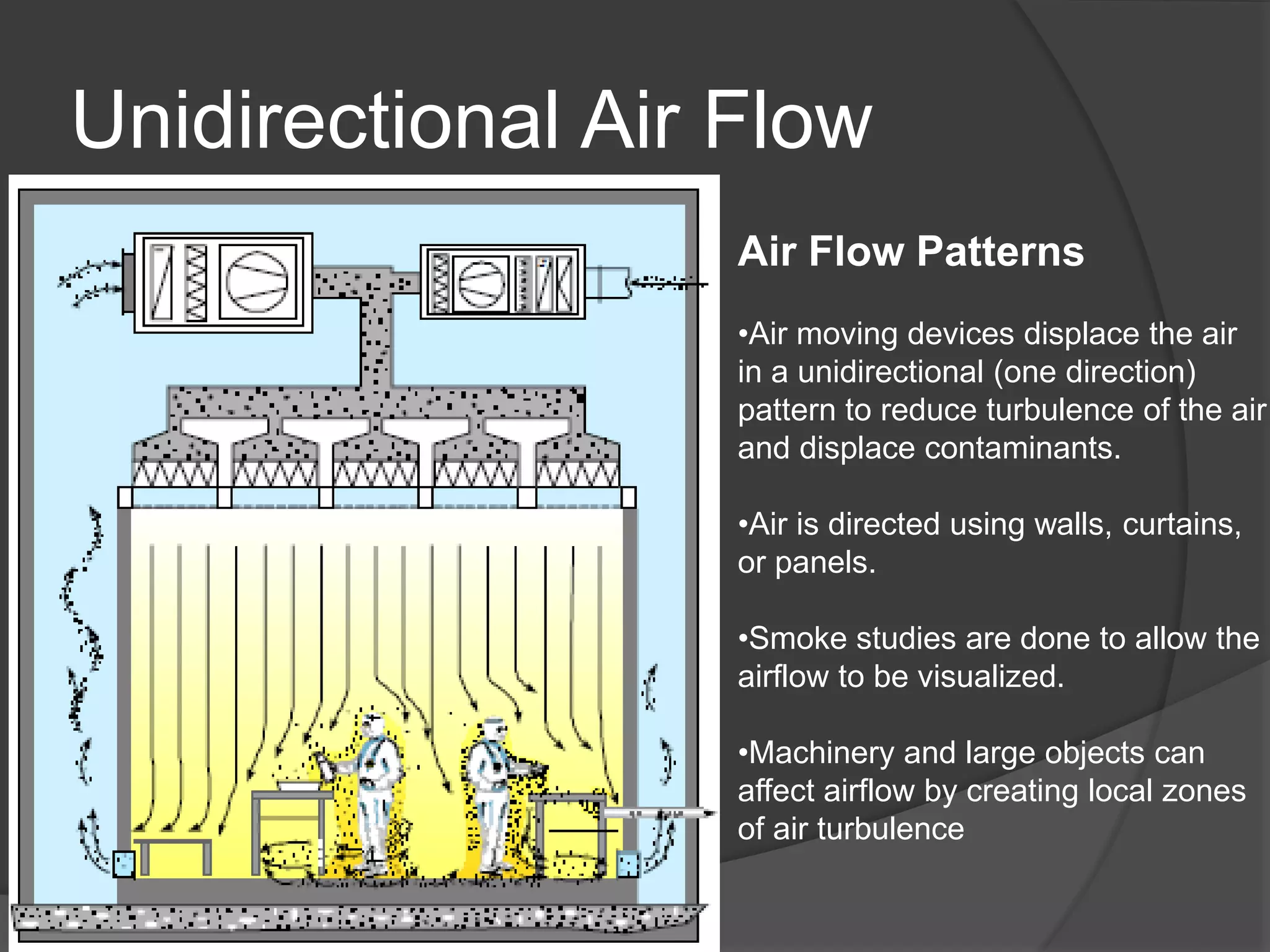
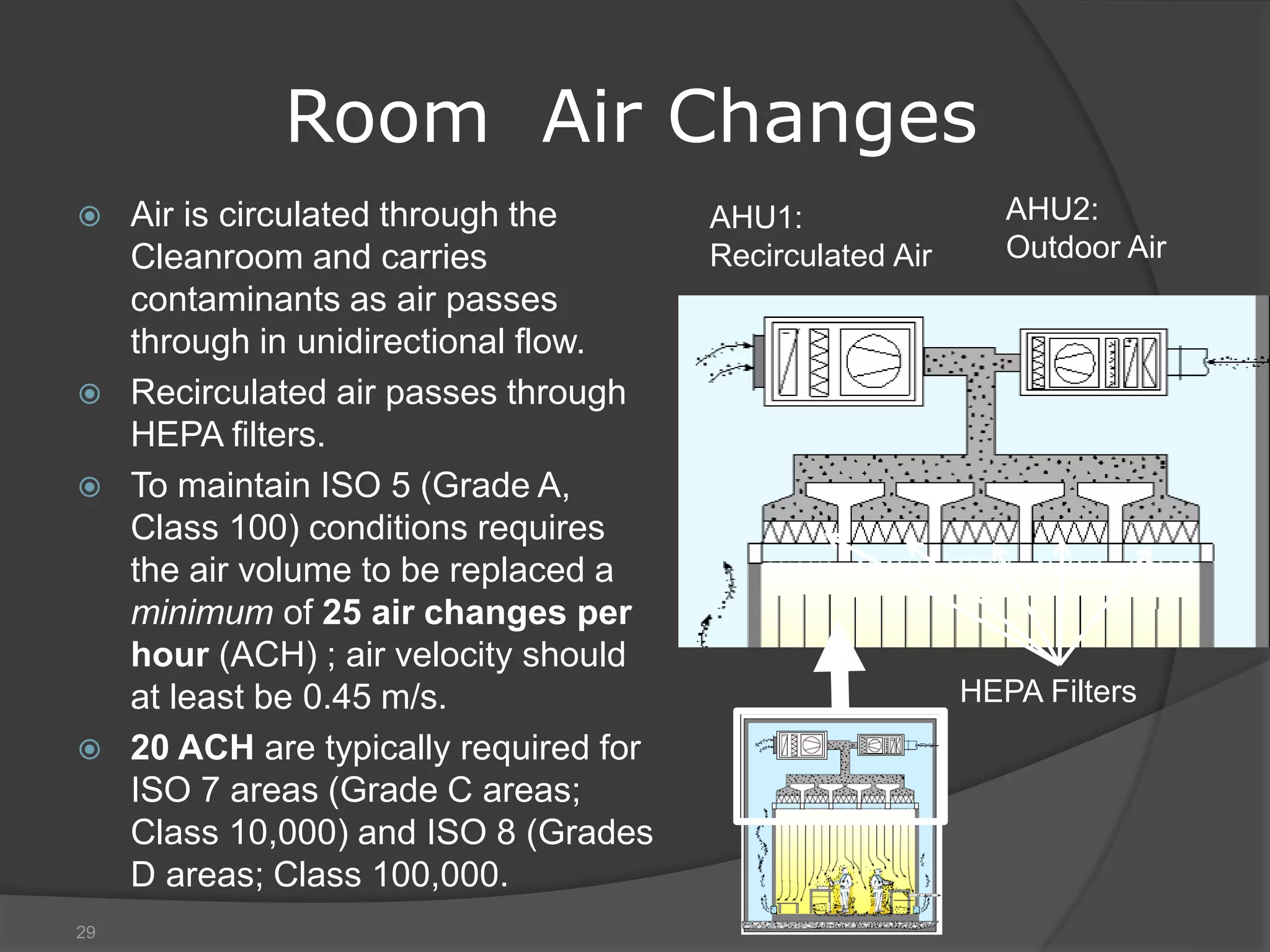
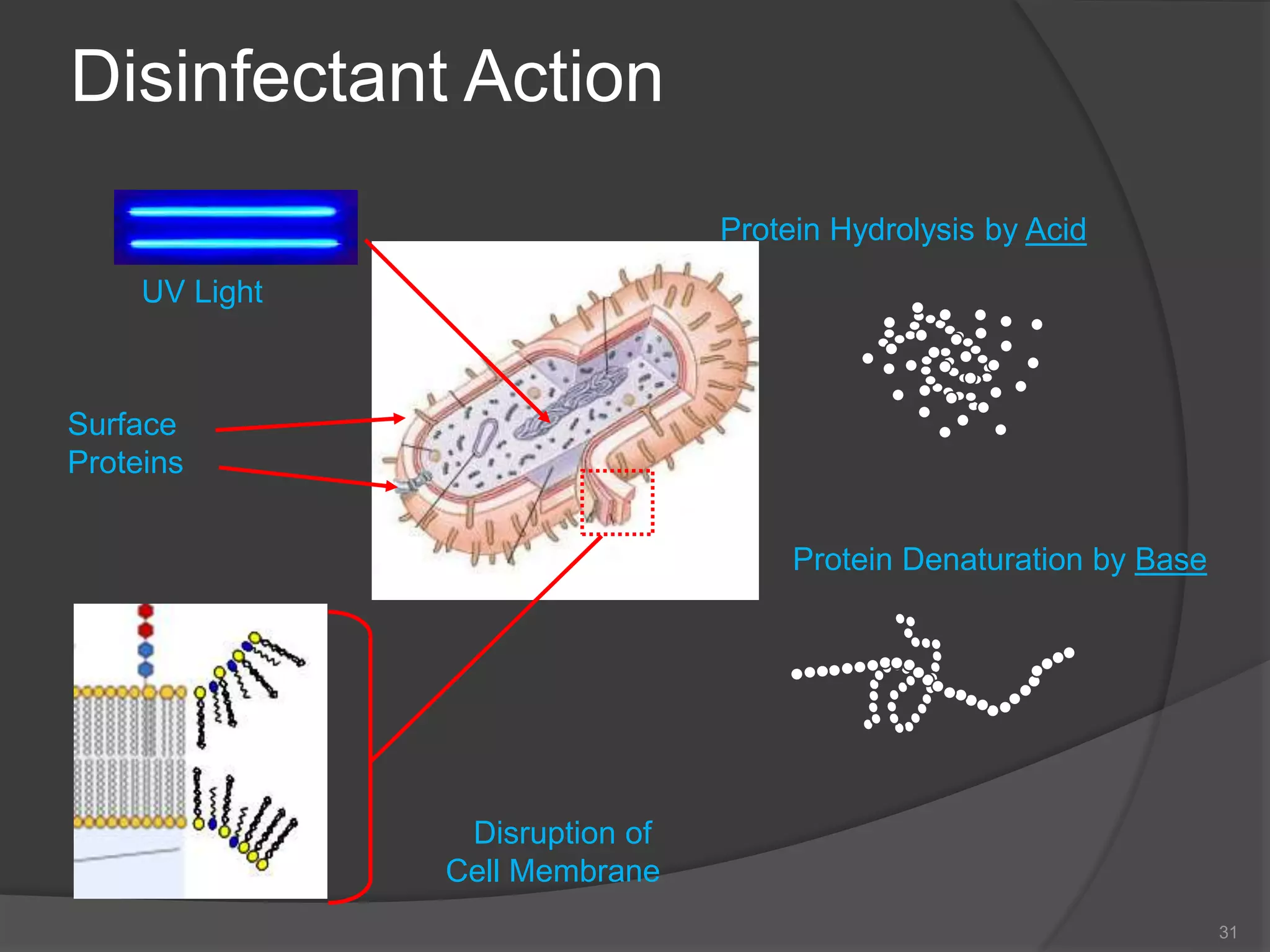
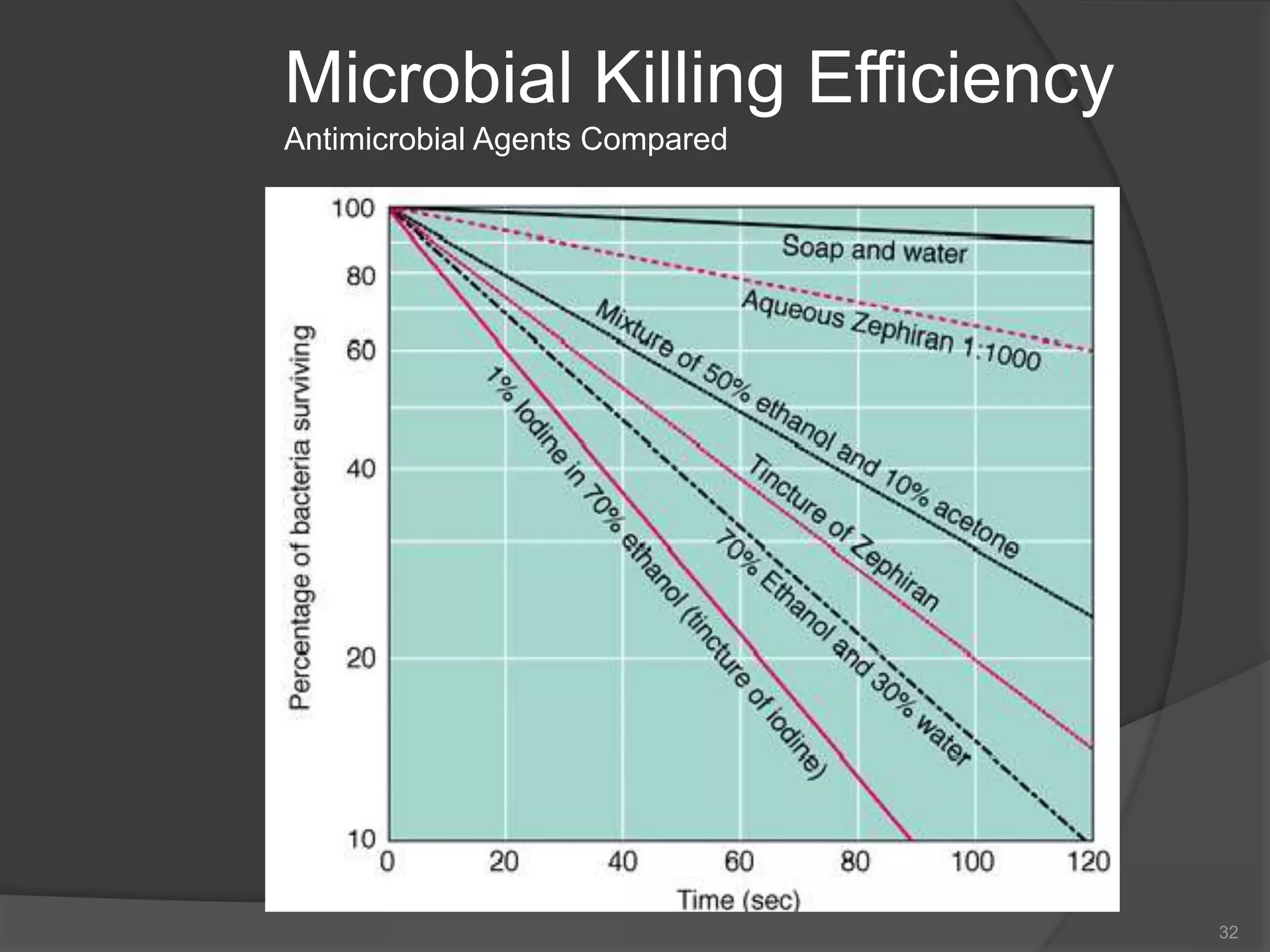
The document provides an overview of aseptic practices and microbiology basics. It discusses:
- Definitions of aseptic, sterile, and related terms.
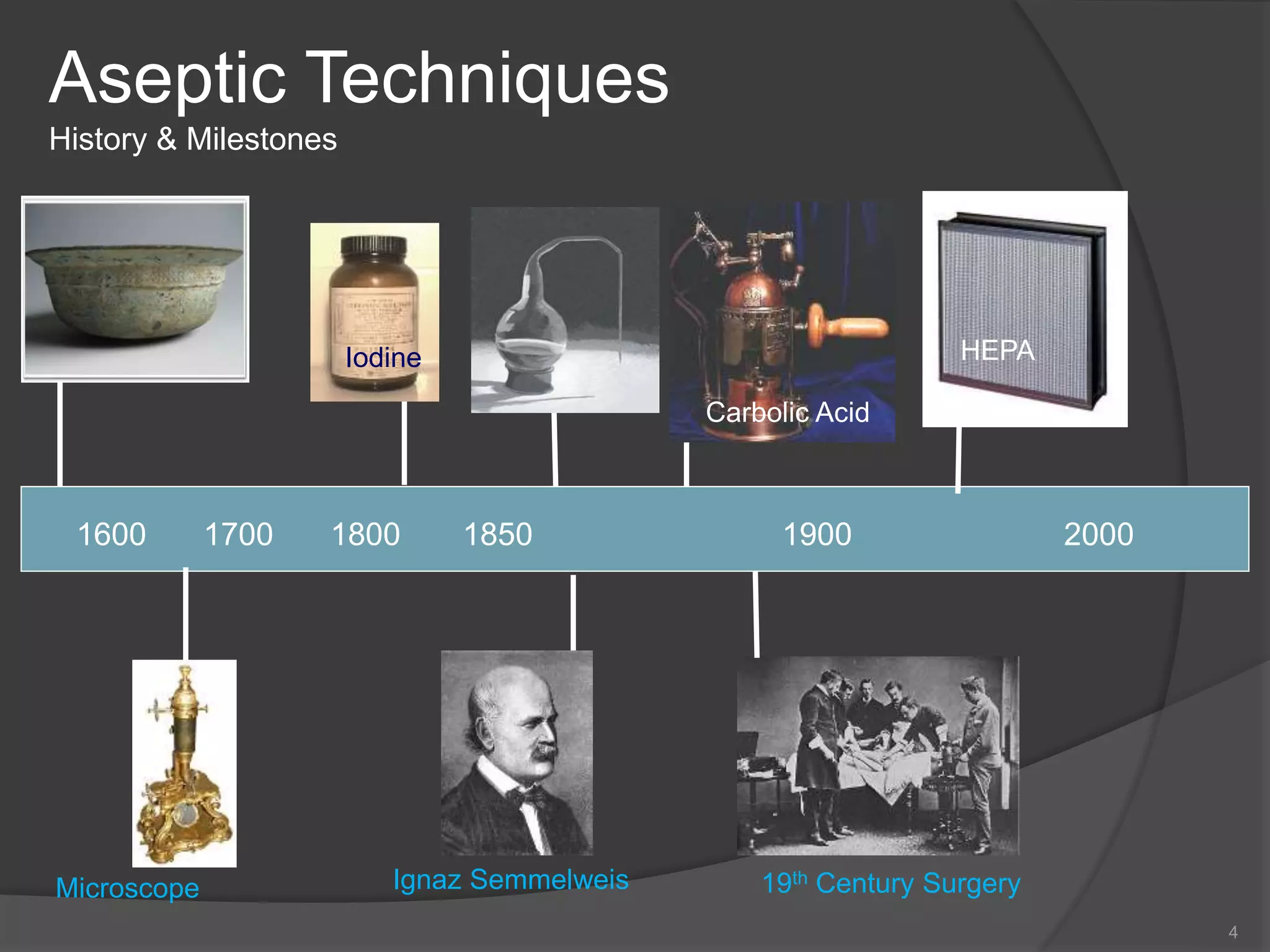
- A brief history of aseptic techniques and milestones like the development of the microscope, iodine, and HEPA filters.
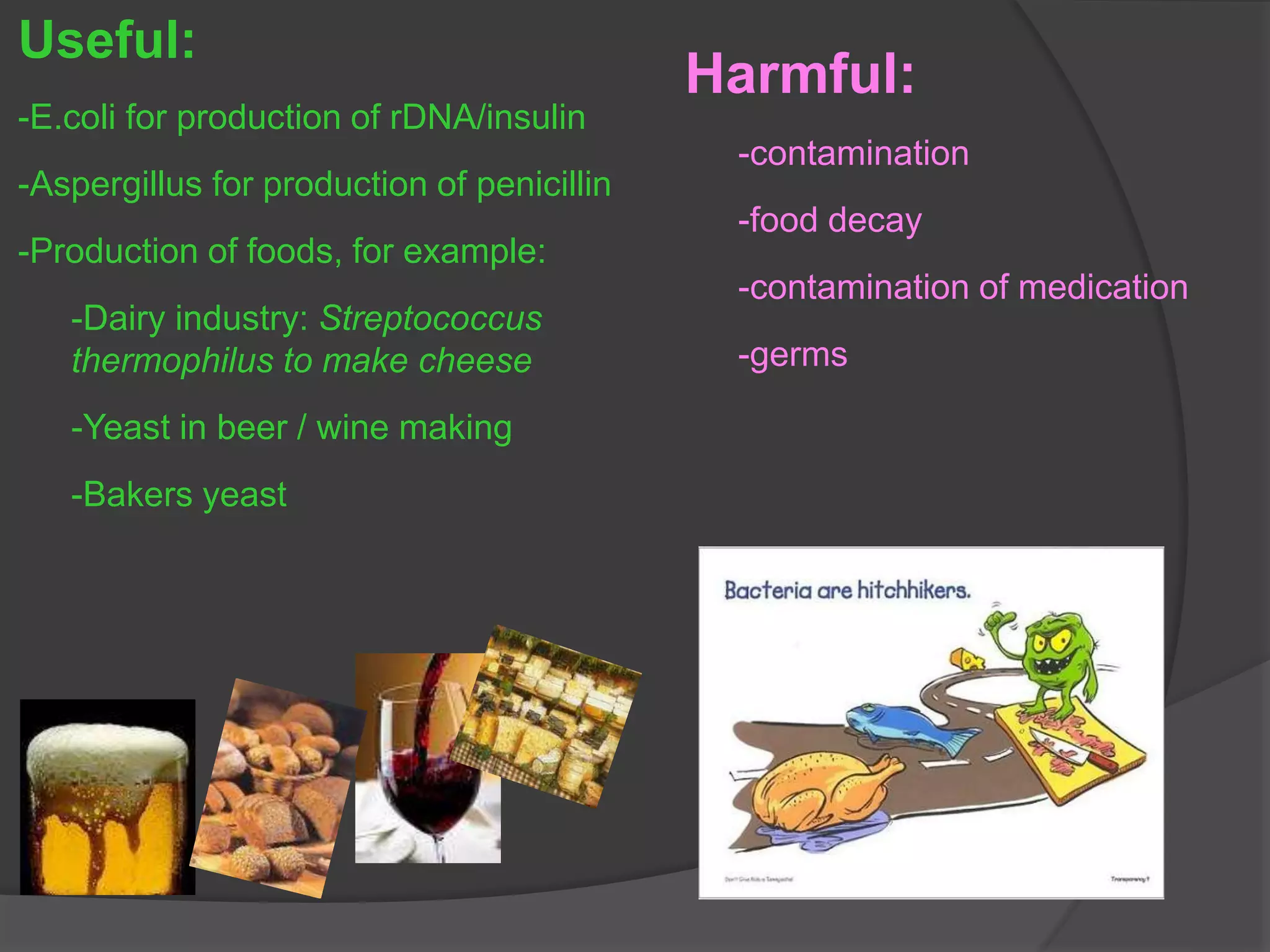
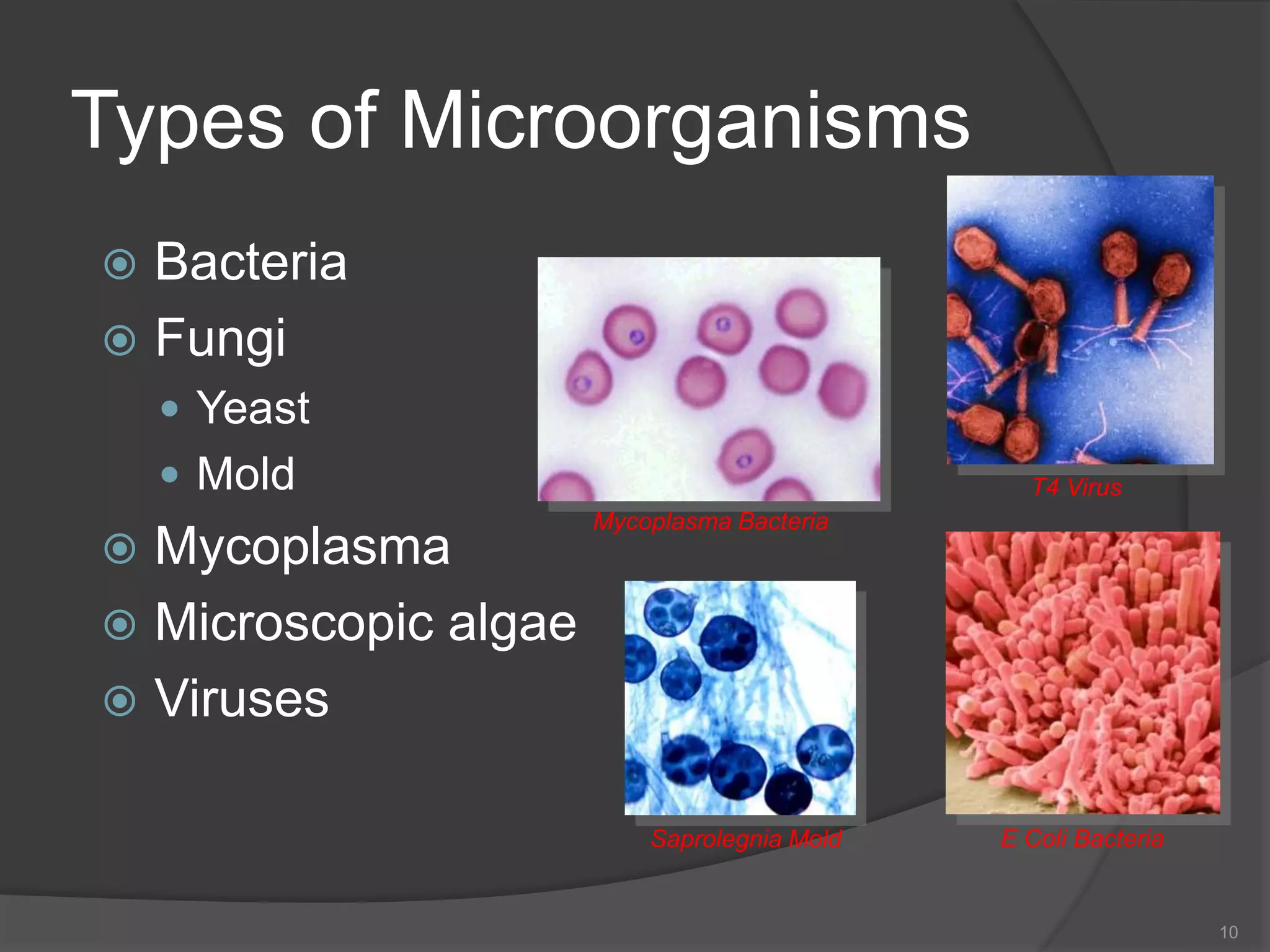
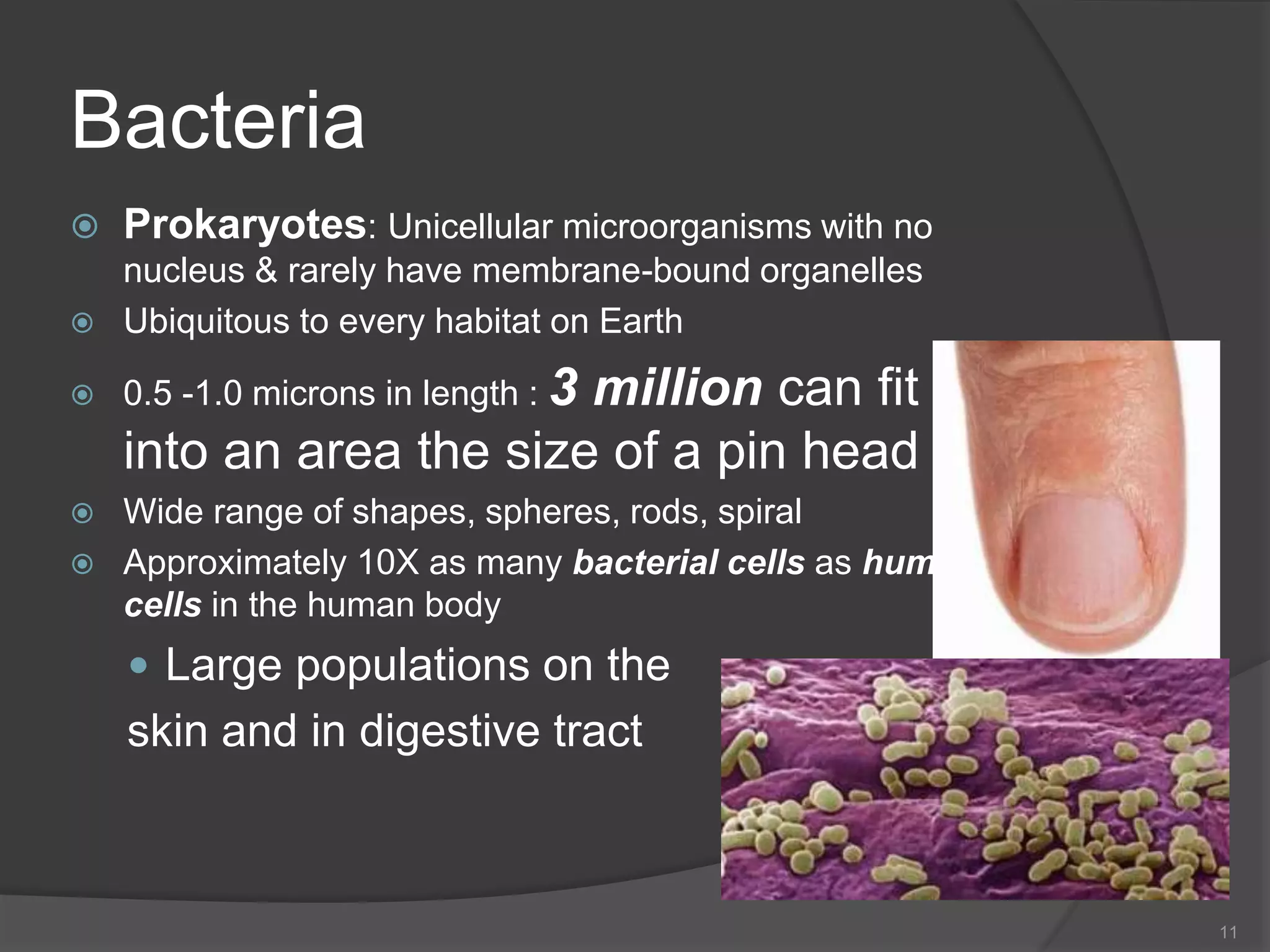
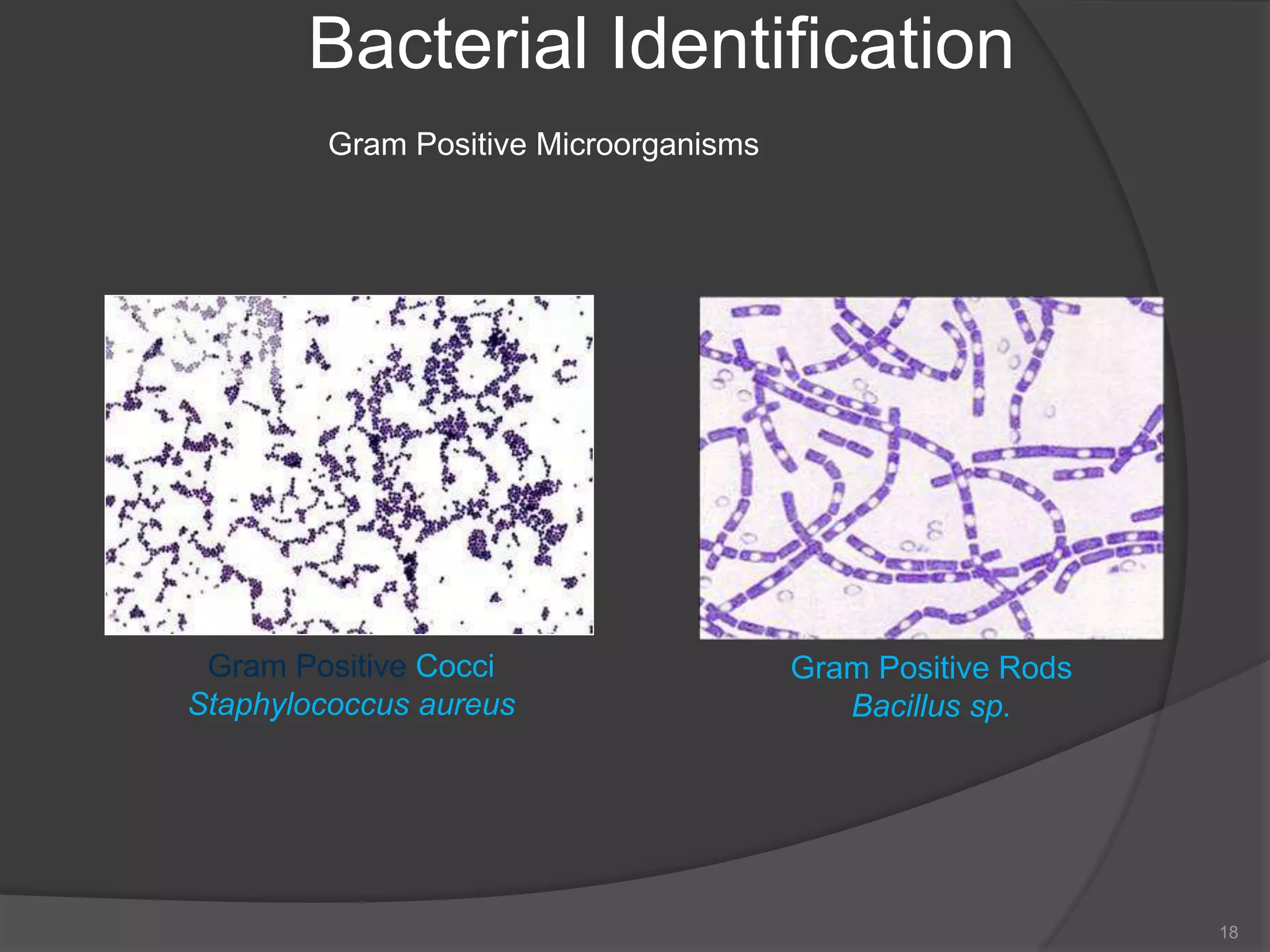
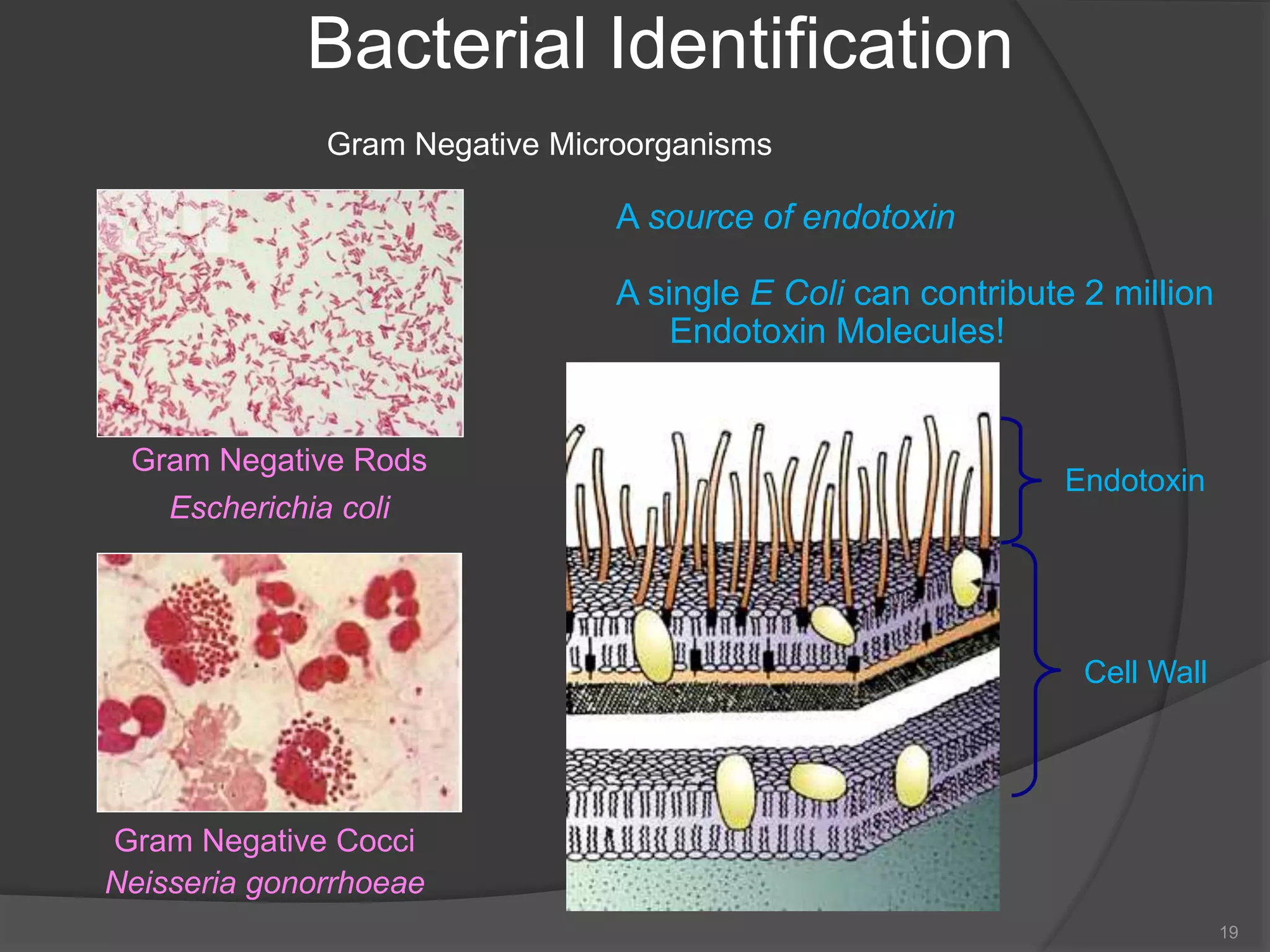
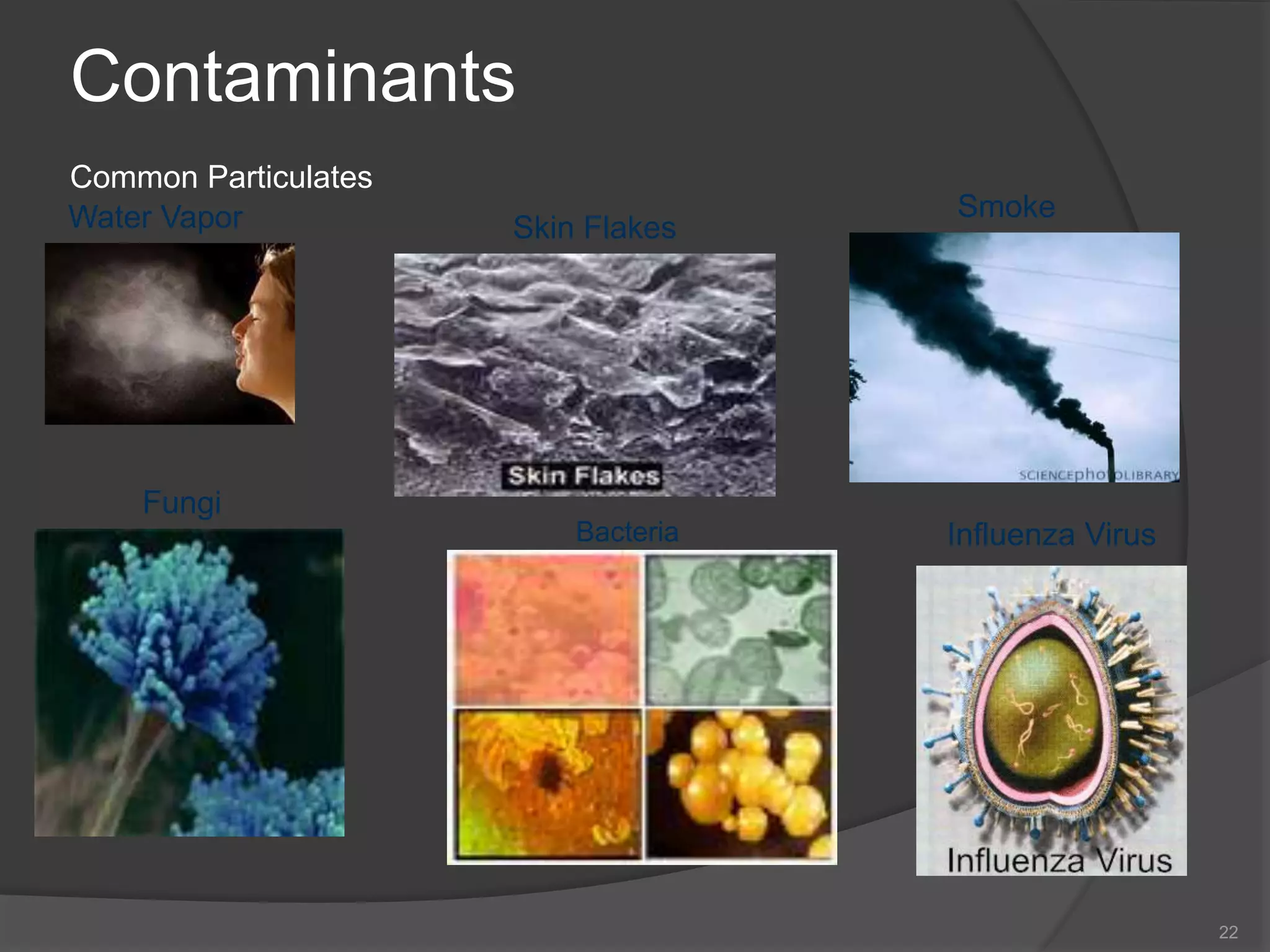
- The importance of microbiology in contamination control and why microbes are studied. It describes common types of microbes like bacteria, fungi, and viruses.
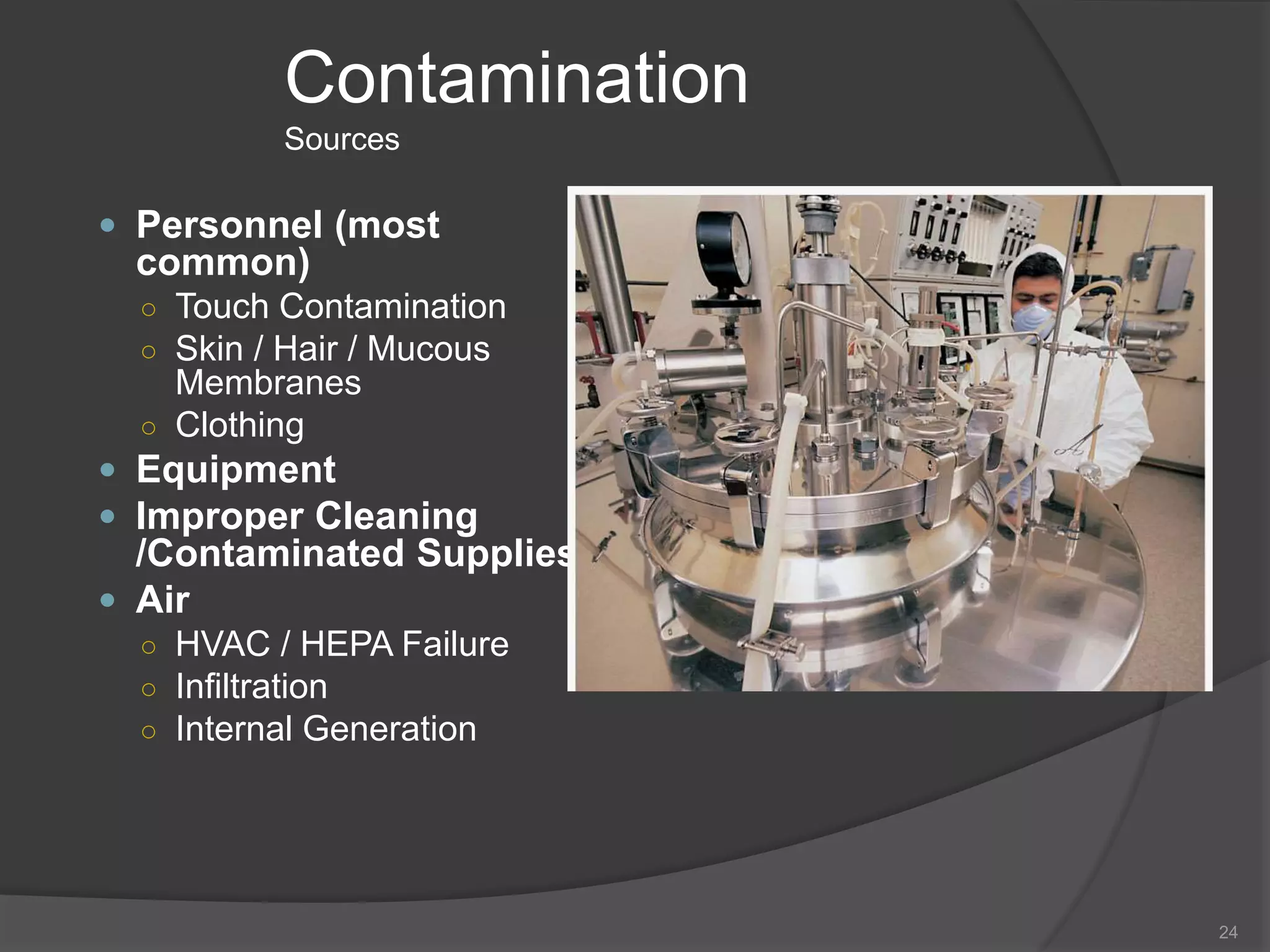
- Sources of contamination and methods for contamination control including cleaning/disinfection, hygiene practices, facilities design with HEPA filters and air flow, and the four pillars of aseptic techniques.
- Personal responsibility in contamination prevention through practices like hand