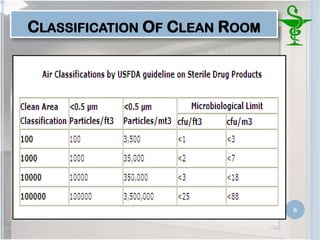
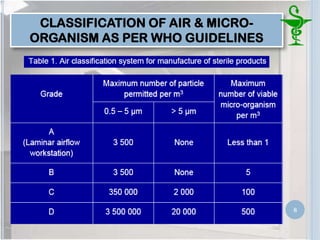
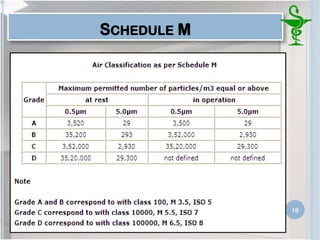
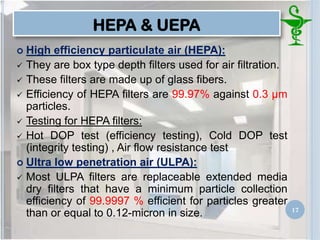
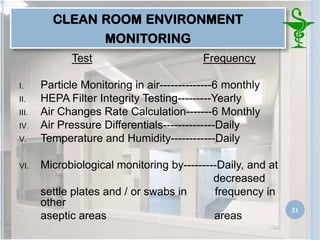
This document provides an introduction to pharmaceutical clean rooms. It discusses the purpose of clean rooms which is to promote successful cleanroom operations and ensure safety. Clean rooms are classified according to international standards based on the number of permitted particles per cubic meter of air. Sources of contamination are discussed as well as methods for contamination control including personnel control, environmental control, and atmospheric monitoring. The conclusion states that the main purpose of a clean room is to prevent contamination of products and ensure quality according to good manufacturing practices.