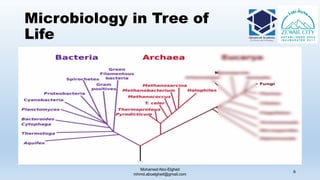
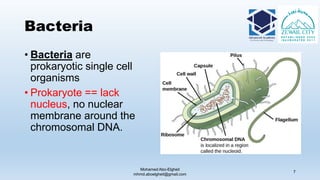
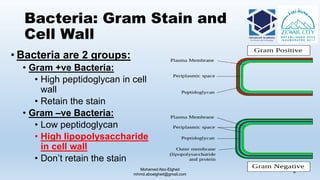
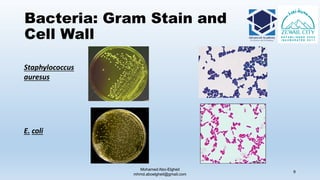
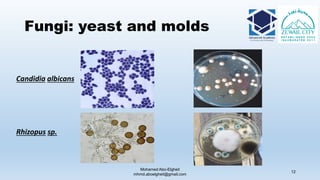
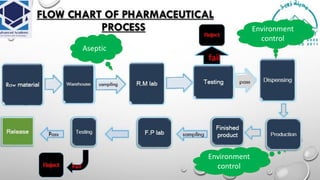
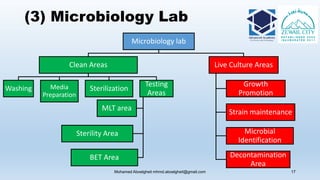
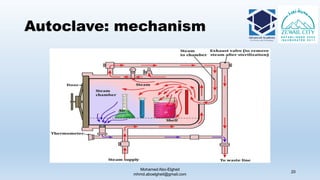
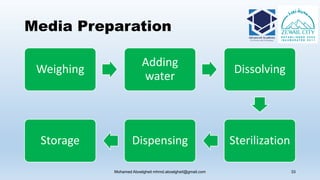
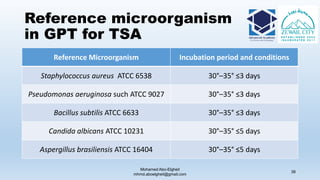
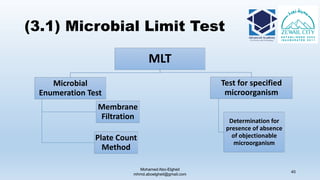
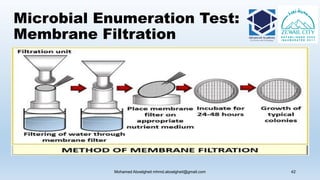
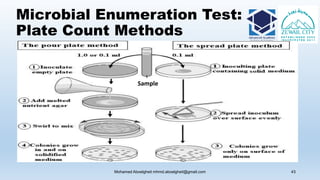
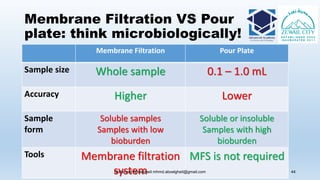
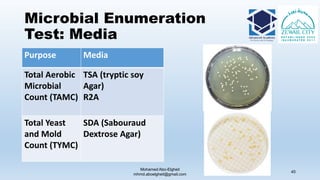
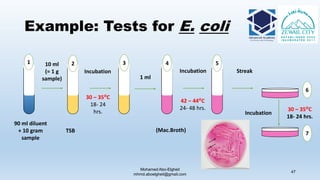
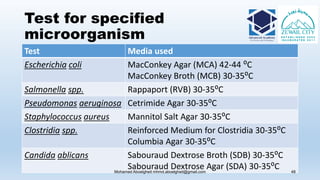
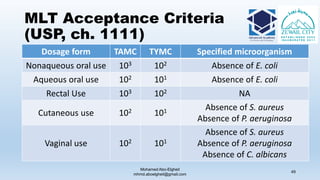
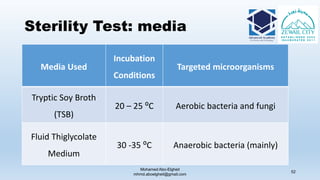
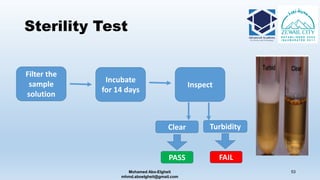
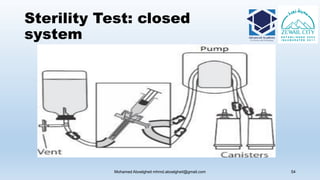
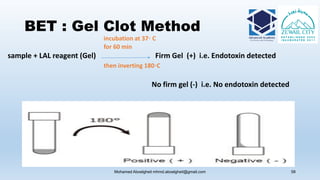
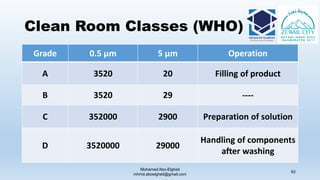
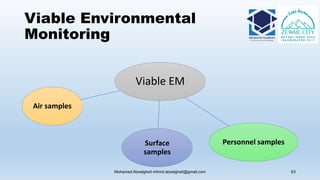
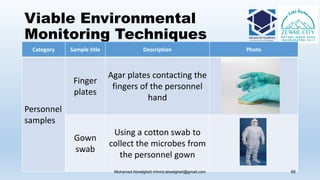
The document is a comprehensive lecture on pharmaceutical microbiology, outlining the role of microbiology labs in the pharmaceutical industry, microbiological tests, and the career path for microbiologists. It covers key concepts such as aseptic techniques, types of microorganisms, microbiological analysis, and good manufacturing practices. Additionally, it includes details about the microbiology lab setup, equipment, and various testing methodologies essential for ensuring the safety and efficacy of pharmaceutical products.