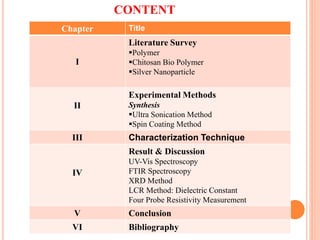
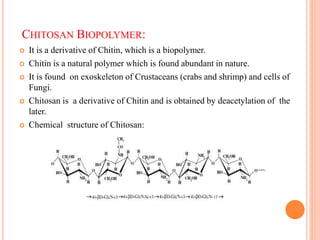
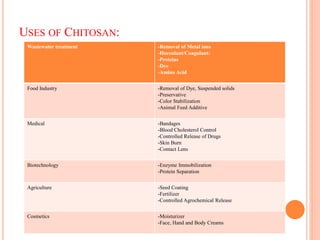
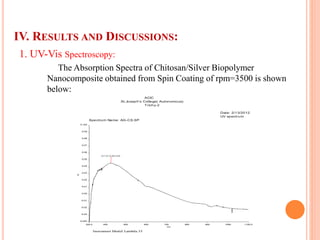
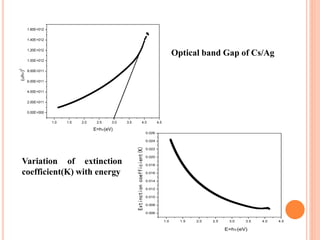
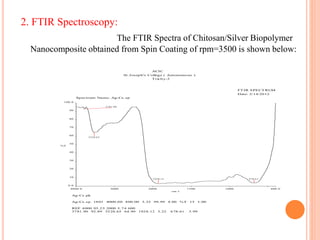
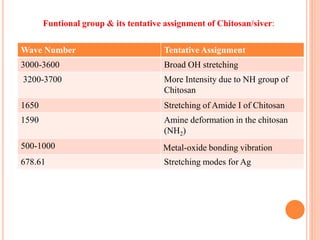
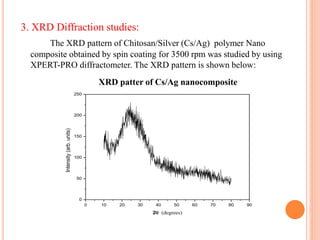
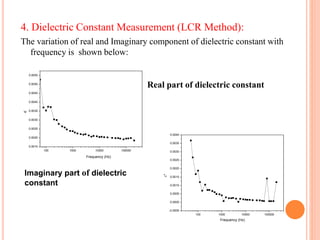
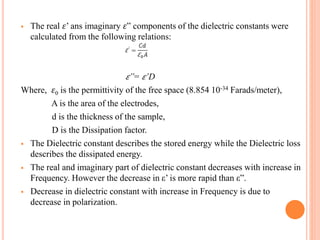
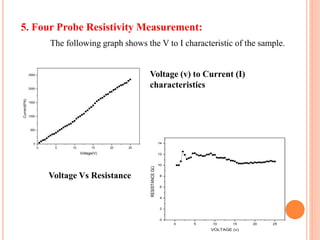
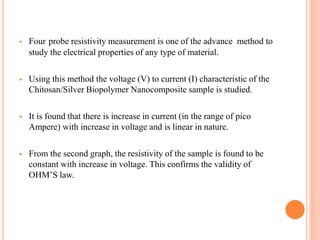
The document summarizes the synthesis and characterization of chitosan/silver biopolymer nanocomposites. Chitosan/silver nanocomposite films were synthesized using ultrasonication and spin coating methods. The films were characterized using UV-Vis spectroscopy, FTIR, XRD, dielectric measurements, and four probe resistivity measurements. The characterization confirmed the presence of silver nanoparticles embedded in the chitosan polymer matrix. The dielectric constant was found to decrease with increasing frequency. Electrical measurements showed linear current-voltage behavior consistent with Ohm's law. The document concluded the synthesis and characterization techniques provided an understanding of the chitosan/silver nanocomposite materials.