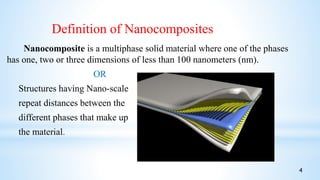
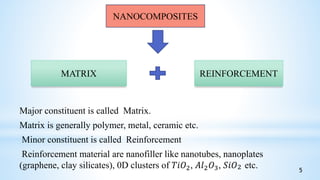
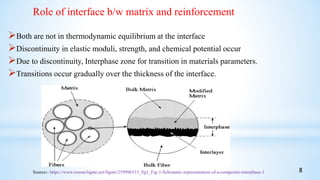
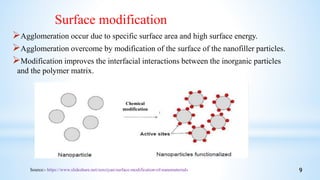
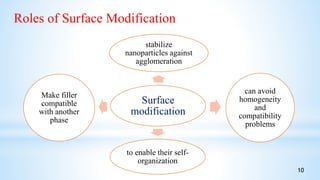
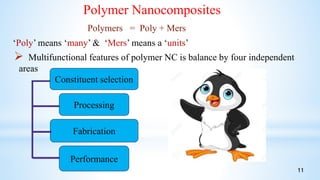
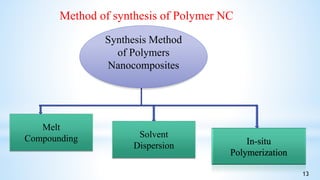
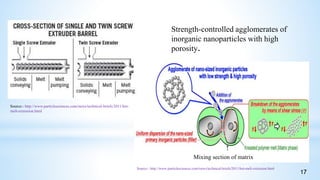
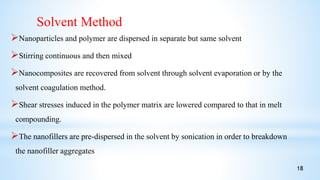
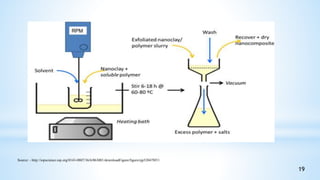
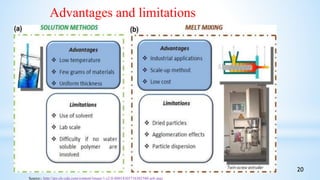
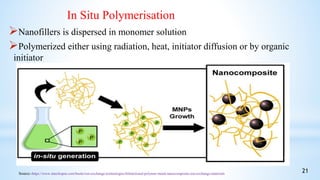
This seminar presentation summarizes polymer nanocomposites. It defines nanocomposites as multiphase solid materials with one phase having dimensions less than 100 nm. The major constituent is the polymer matrix and the minor constituent is nanoscale reinforcement materials like nanotubes, nanoplates, or nanoparticles. The advantages of nanoscale fillers over conventional fillers include low percolation thresholds, large interfacial areas, and short particle distances. Surface modification of nanofillers is important to prevent agglomeration and improve interfacial interactions. Common synthesis methods for polymer nanocomposites include melt compounding, solvent processing, and in situ polymerization. Polymer nanocomposites provide enhanced properties compared to


![‘Nanocomposites’ = Nano + Composites
‘Nano’ means very small ratio, billionth part of one
[ 1nano = 10−9
]
‘Composites’ are combinations of more than two,
difference phase materials
What are nanocomposites?
3](https://image.slidesharecdn.com/seminaronnanocomposites-180325161322/85/Seminar-on-nanocomposites-3-320.jpg)