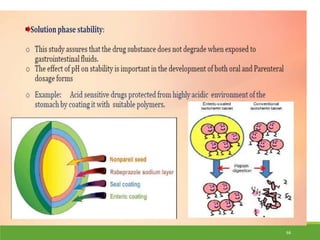
This document provides an overview of pharmacology and pharmaceutical dosage forms. It discusses the scope and objectives of understanding how pharmaceutical additives and dosage forms influence drug performance. The objectives are to understand various dosage forms and manufacturing techniques, considerations in dosage form development, and how to formulate and evaluate solid, liquid and semisolid dosage forms. It also discusses basic pharmacology concepts like how drugs work, are absorbed and distributed in the body, and the objective of drug therapy to deliver drugs to the right site of action. Preformulation studies are introduced, which involve characterizing the physical and chemical properties of drug substances and understanding how these properties impact dosage form development and stability.