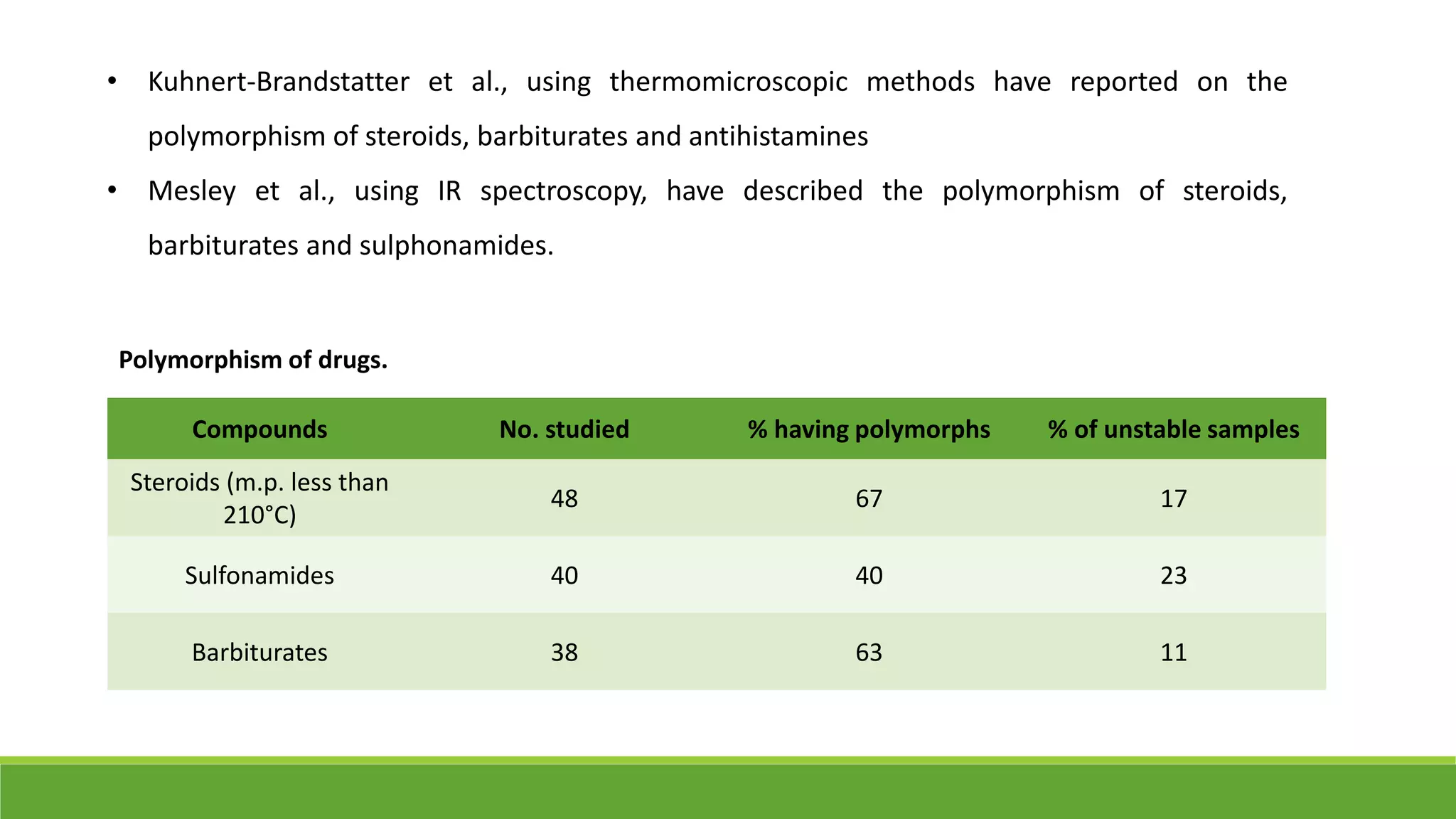
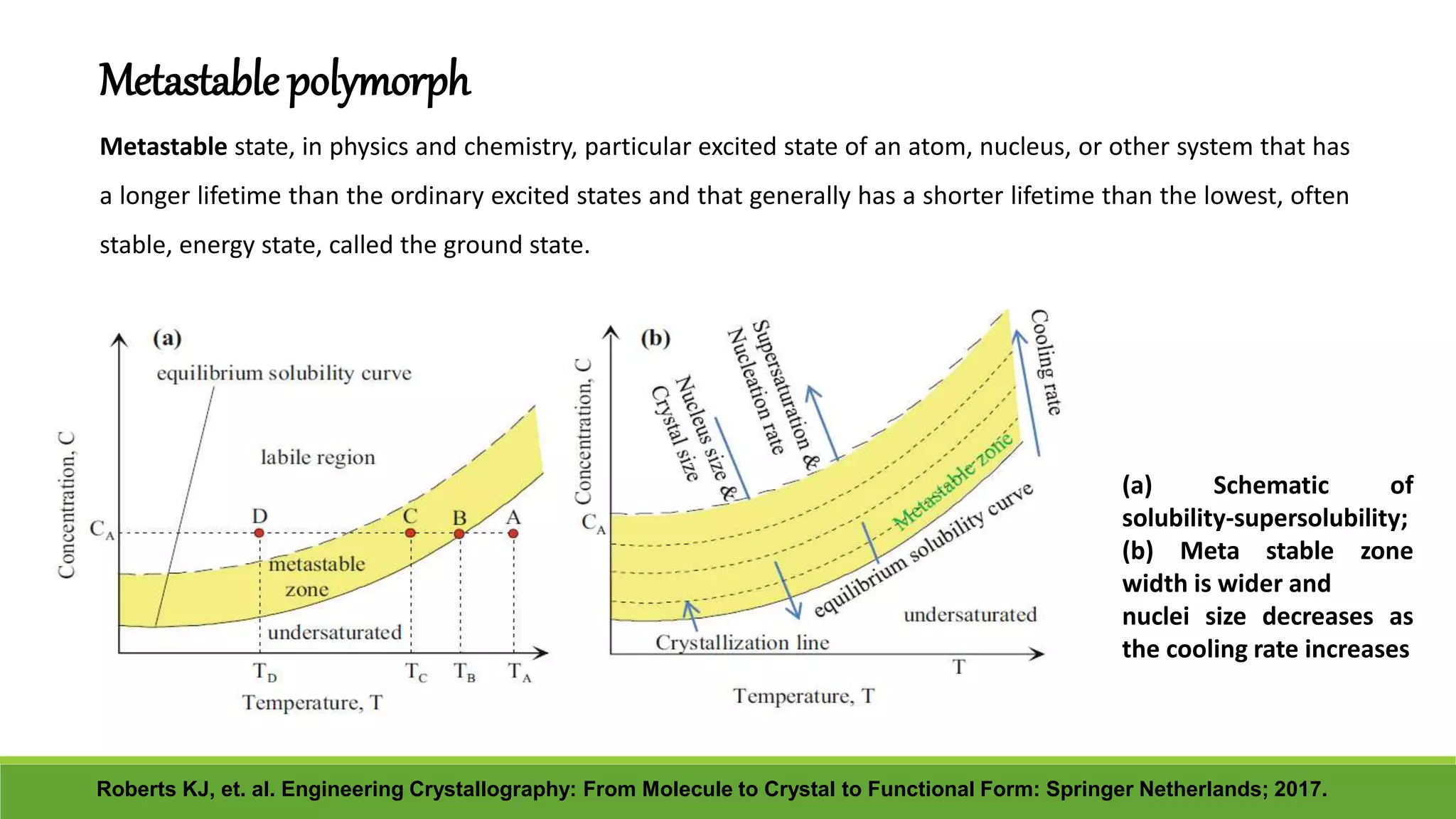
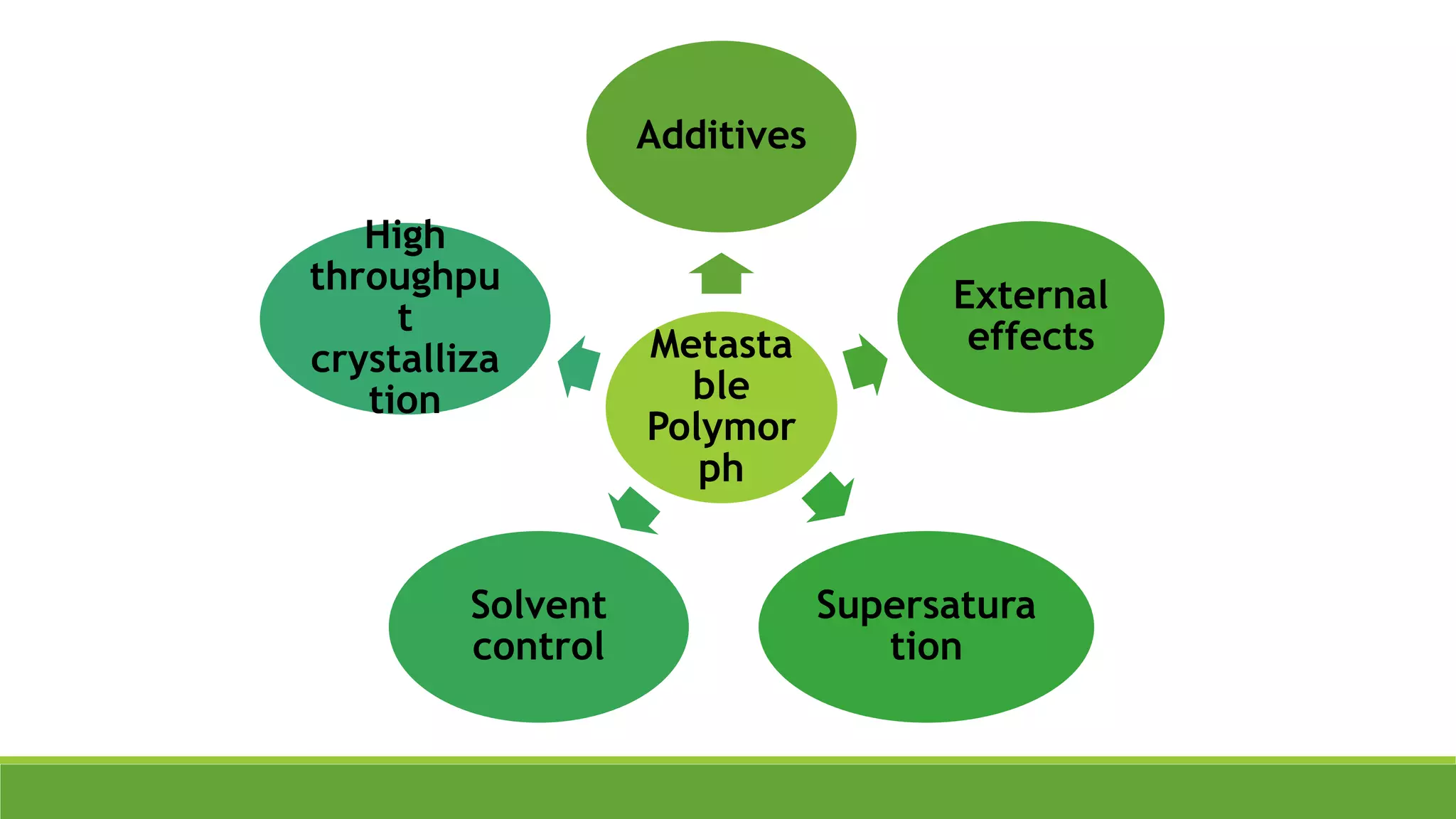
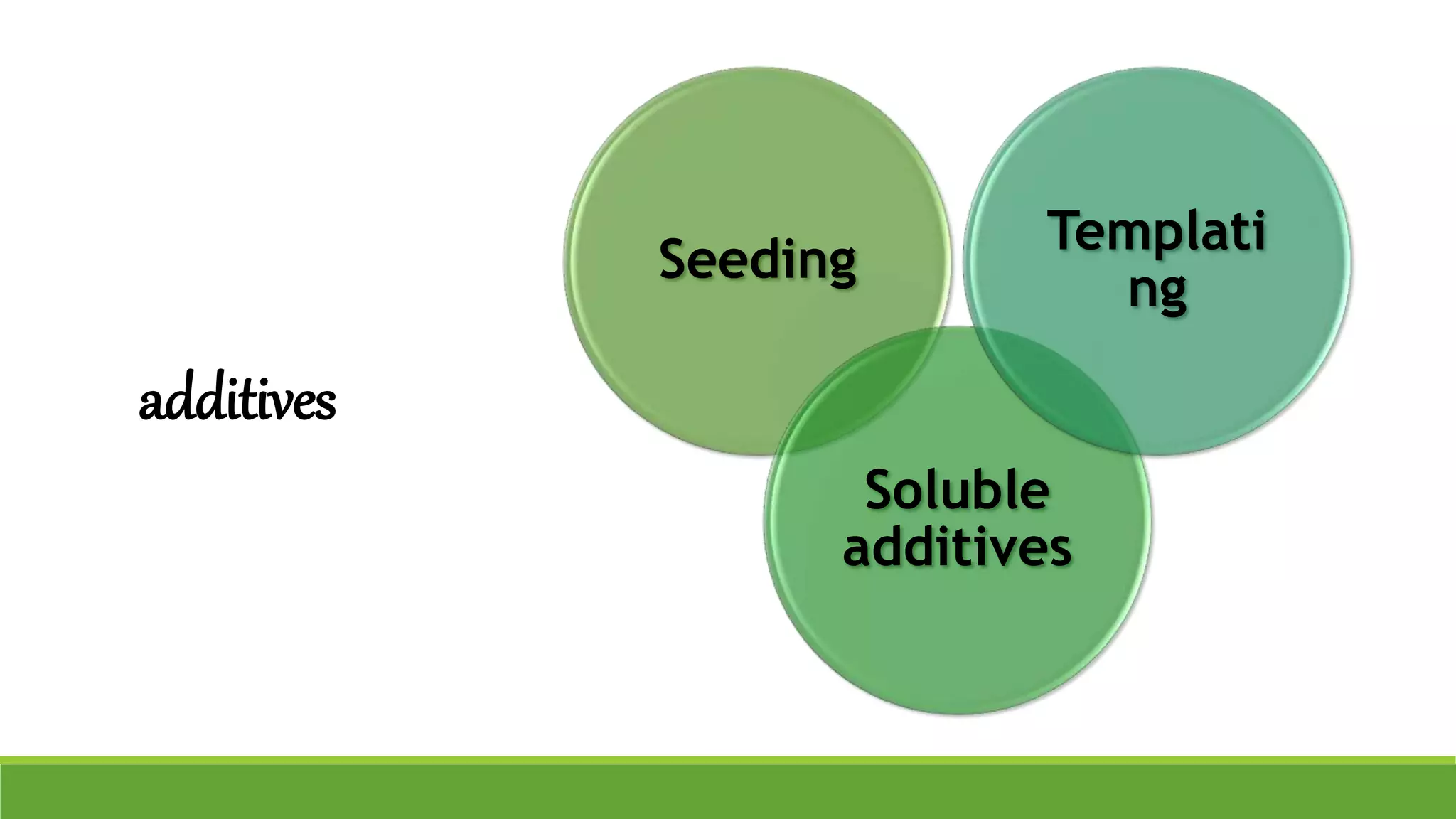
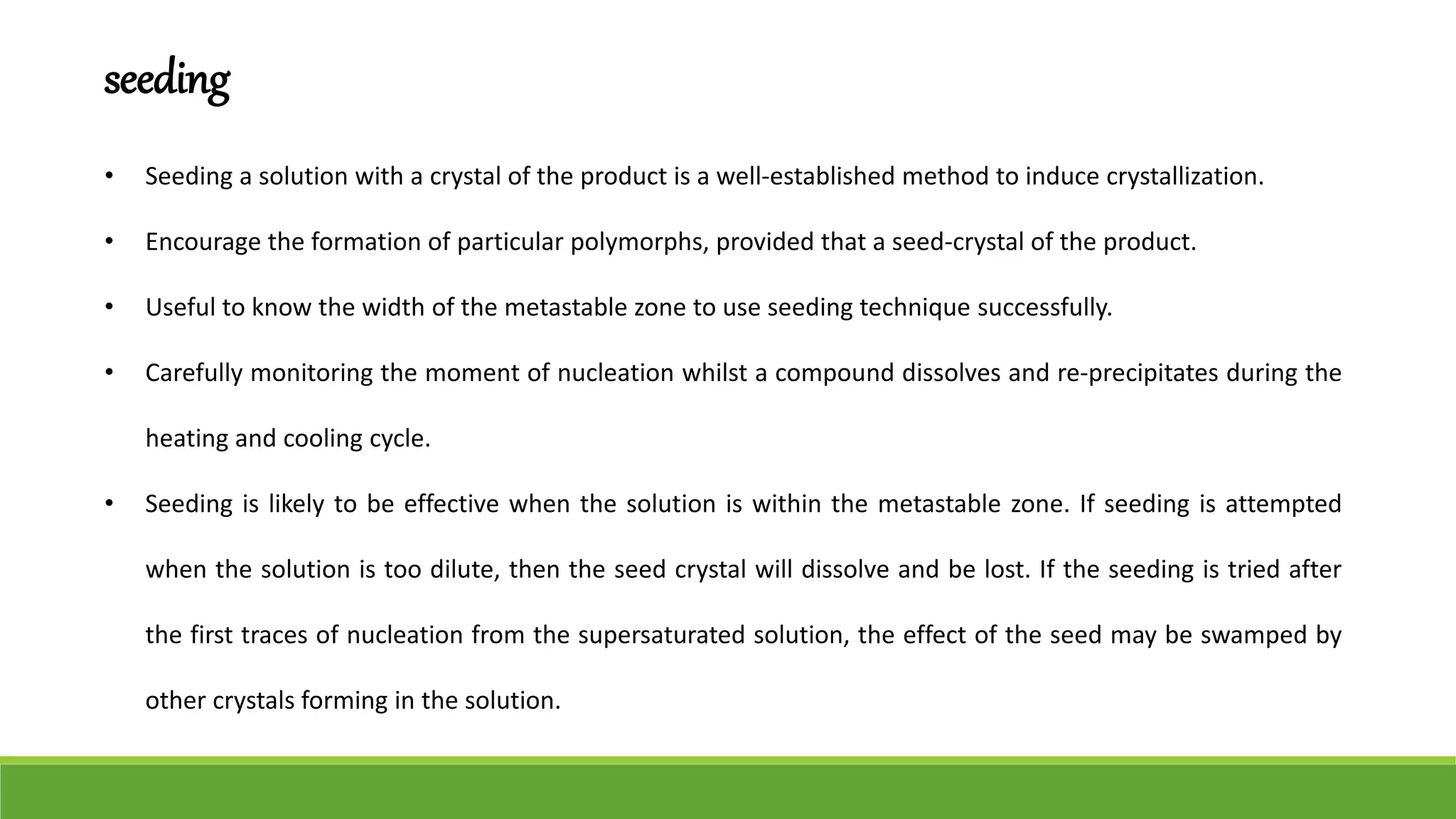
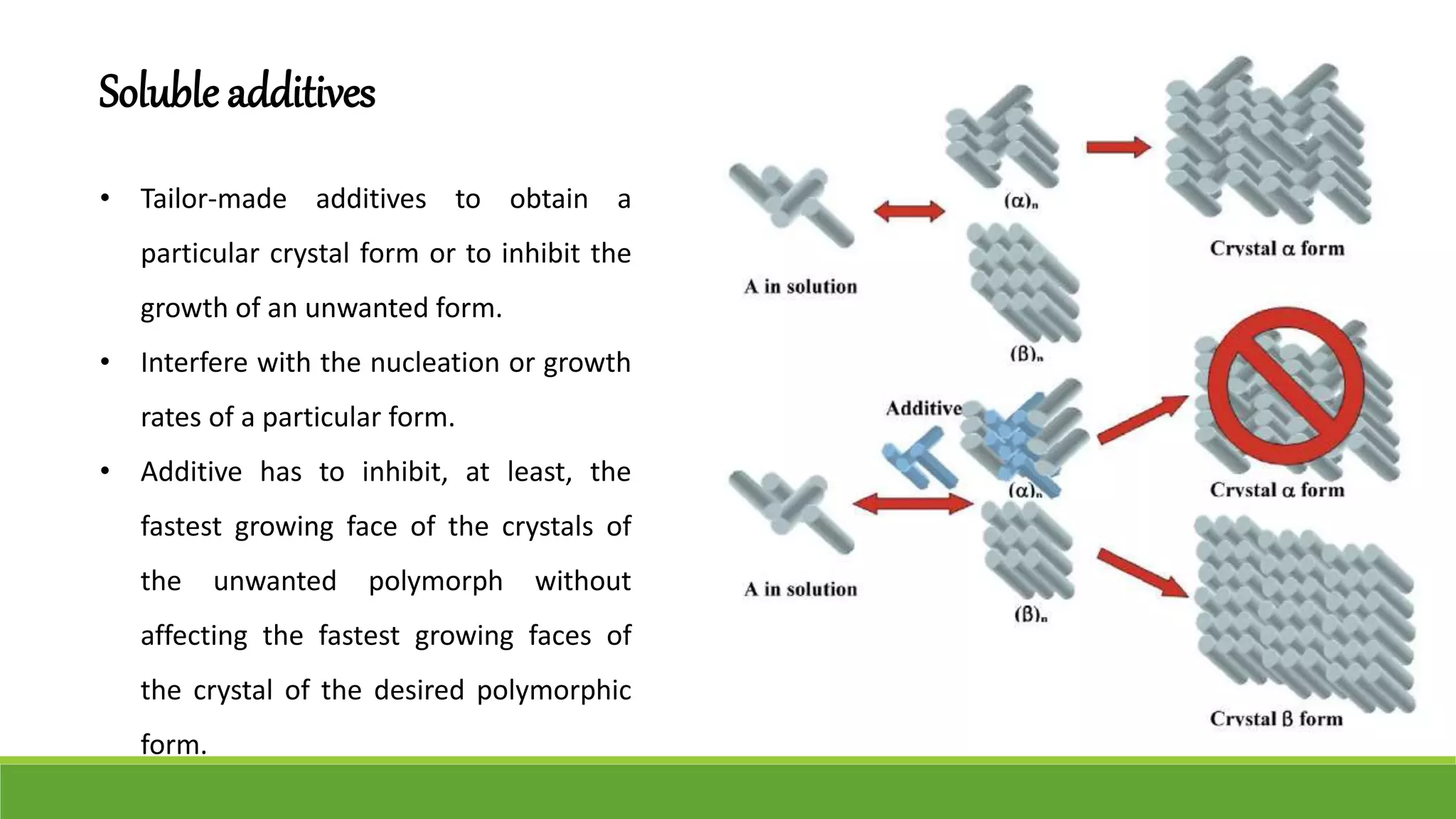
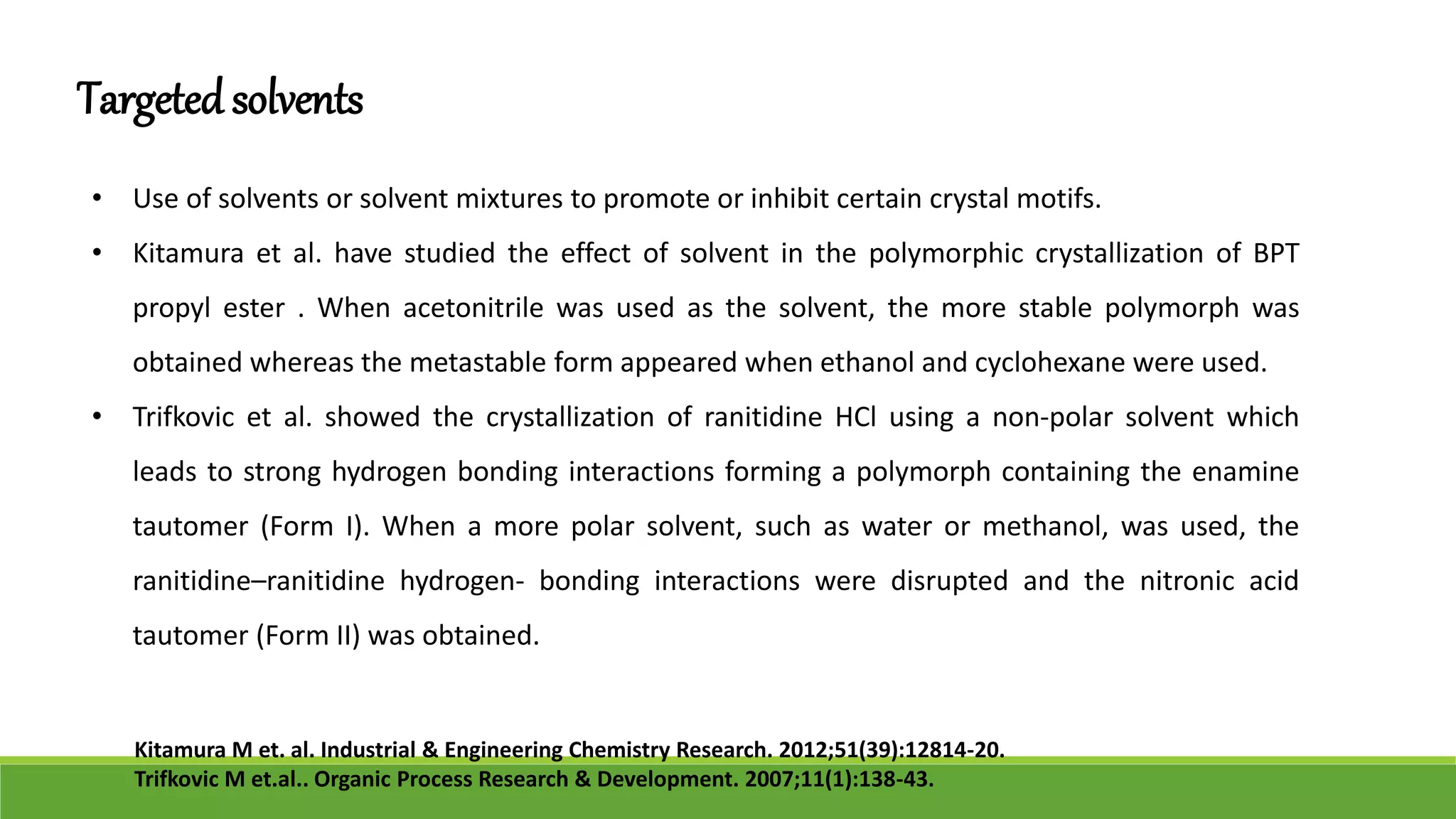
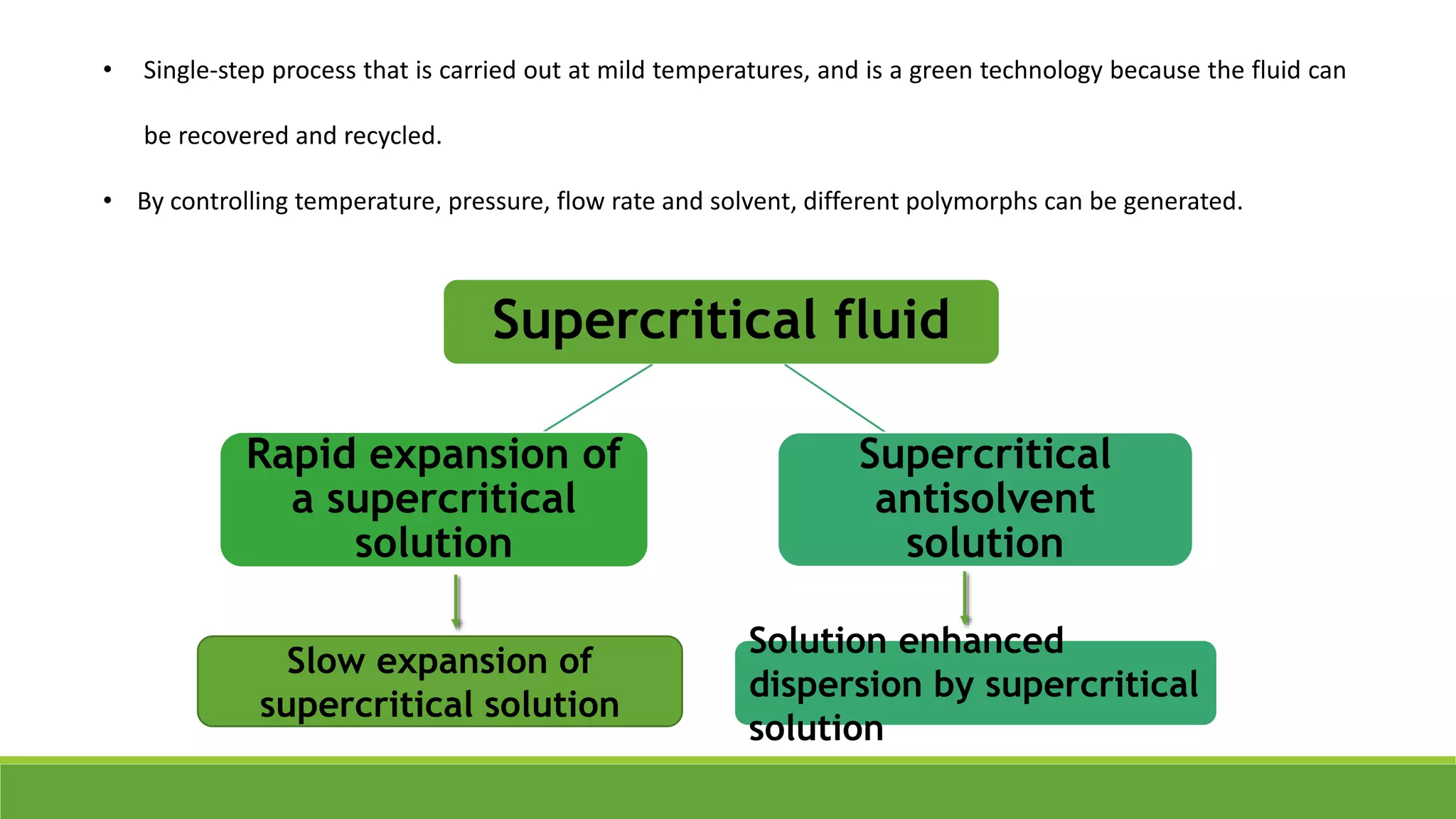
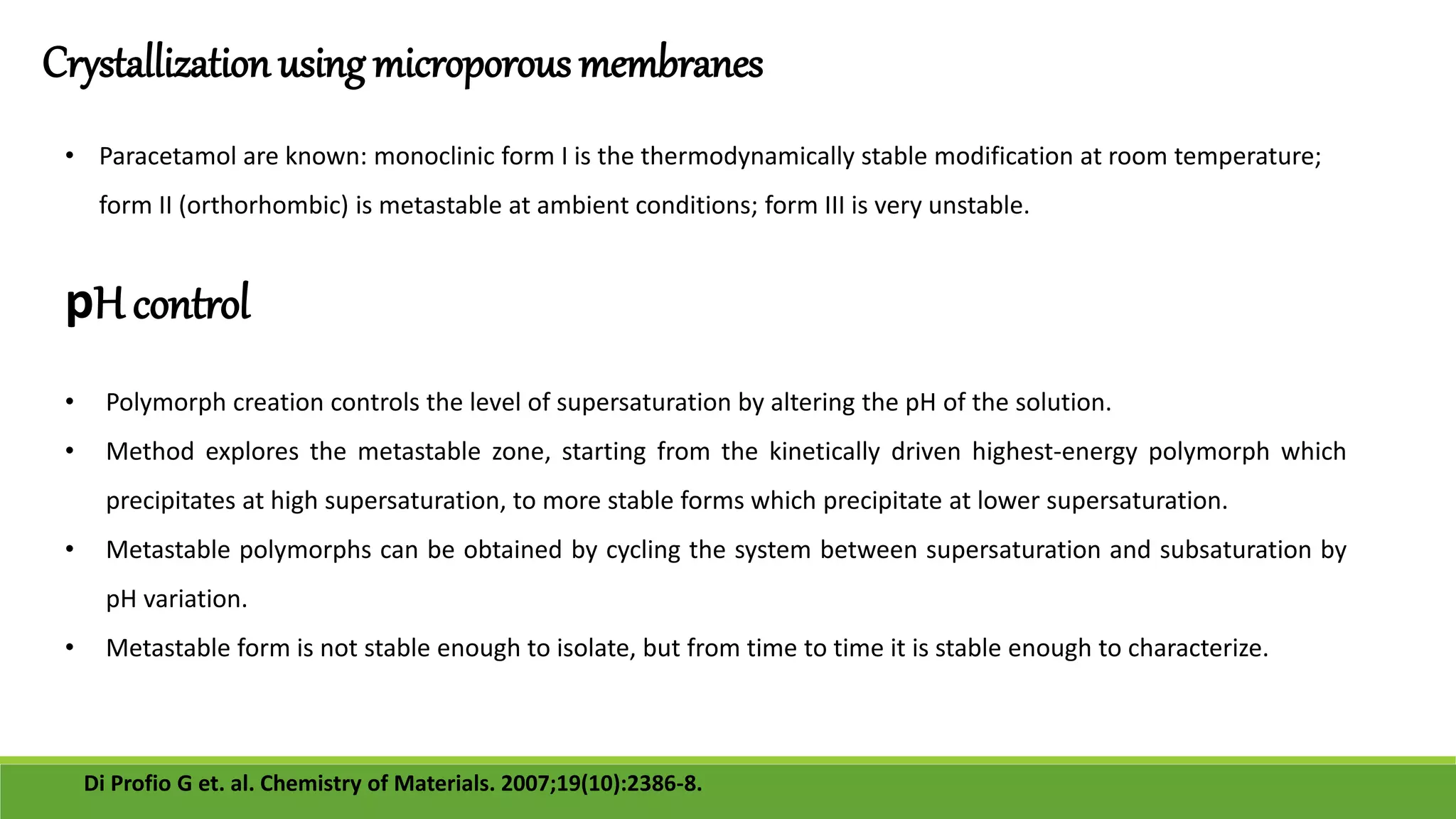
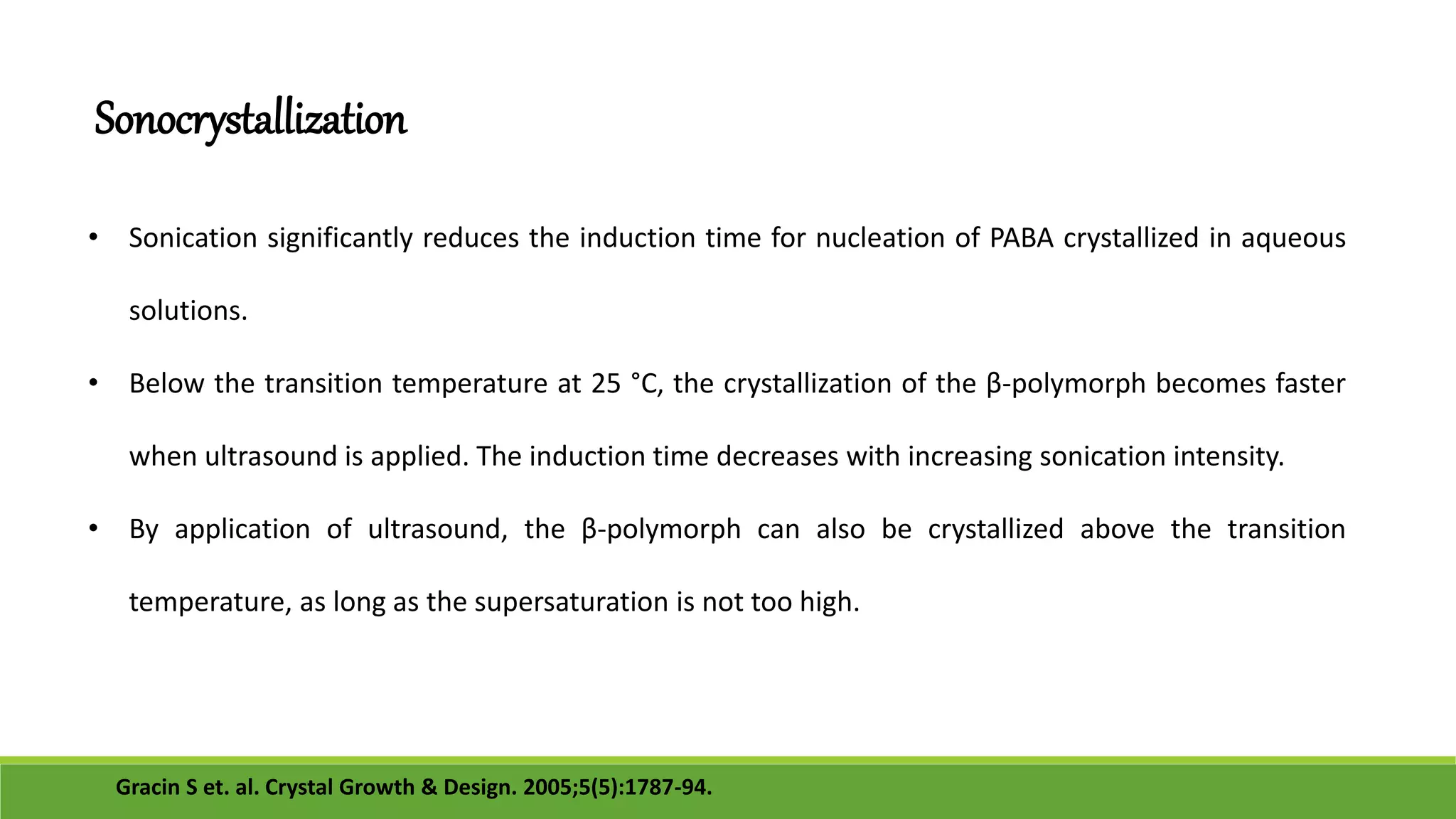
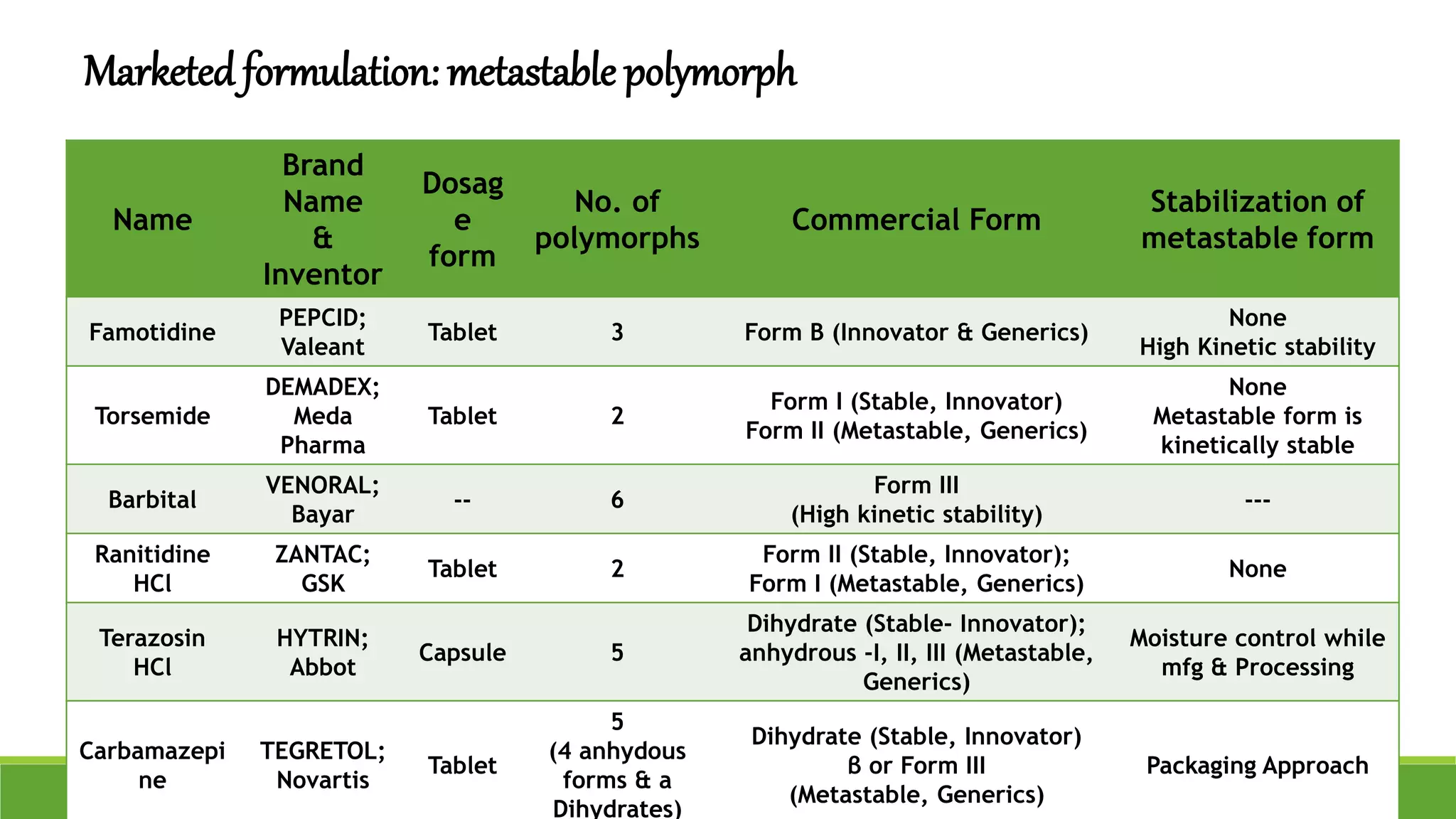
The document discusses the use of metastable polymorphs to enhance oral bioavailability. It begins by defining polymorphism as the ability of a compound to crystallize in more than one distinct crystal structure. Metastable polymorphs are excited crystalline states that have longer lifetimes than ordinary excited states but shorter than the ground state. Using metastable polymorphs can improve properties like solubility and bioavailability. Several techniques to produce metastable polymorphs are described, like seeding, additives, and solvent control. Case studies demonstrate how metastable forms of drugs like famotidine and terazosin hydrochloride were approved generically. Regulatory considerations for showing sameness to the reference listed drug are also covered.