1. Preformulation studies involve characterizing the physical and chemical properties of a new drug substance to help design an optimal dosage form. This includes determining solubility, stability, and compatibility with excipients.
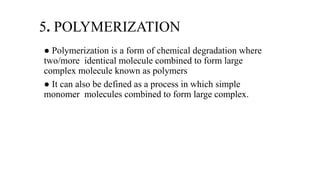
2. The physical properties studied include polymorphism, solubility, and organoleptic characteristics. Chemical properties like hydrolysis, oxidation, reduction, racemization, and polymerization are analyzed to understand the drug's degradation pathways.
3. Understanding the preformulation properties helps ensure development of a safe, stable, and effective dosage form to deliver the drug to patients.

![INTRODUCTION
● Preformulating involves the application of biopharmaceutical
principles to the physicochemical parameters of a drug with the goal of
designing an optimum drug delivery system. [1]
● Each drug substance has intrinsic chemical and physical
characteristics that must be considered before development of a
pharmaceutical formulation.
● So, the characterization of drug molecule is very important step at the
preformulating phase of product development.](https://image.slidesharecdn.com/m-230817053522-073823f7/85/formulation-Development-2-320.jpg)
![DEFINITION
● The word pre - formulation refers to the step to be undertaken
before formulation. It includes determination of physical and
chemical properties of drug substances. Preformulating main goal
is to develop a new drug with safe, stable and effective[1]](https://image.slidesharecdn.com/m-230817053522-073823f7/85/formulation-Development-3-320.jpg)


![IMPORTANCE OF
PREFORMULATION
● To form desired quality dosage form.[2]
● To develop an optimum dosage form.
● For targeted drug delivery system.
● To minimize cost of finished product.
● To minimize errors in formulations of dosage form.](https://image.slidesharecdn.com/m-230817053522-073823f7/85/formulation-Development-6-320.jpg)
![GOALS AND OBJECTIVES
● To establish the physio-chemical parameters of a new drug
entity.[2]
● To determine its kinetics and stability.
● To establish its compatibility with common excipients
● To develop optimal drug delivery system](https://image.slidesharecdn.com/m-230817053522-073823f7/85/formulation-Development-7-320.jpg)
![PHYSICAL PROPERTIES
1.Organoleptic characteristics:[3]
● Color, odor, taste of the new drug must be recorded.
2.Physical forms:
● Depending on internal structure compounds is classified as
A.Crystalline (Solid)
B. Amorphous (powder form](https://image.slidesharecdn.com/m-230817053522-073823f7/85/formulation-Development-8-320.jpg)

![POLYMORPHISM
● It is the ability of the compound to crystallize as more than one distinct crystalline species
with different internal lattice. [4]
● Different crystalline forms are called polymorphs
● Polymorphs are of 2 types
A. Enantiotropic
B. Monotropic
● The polymorph which can be changed from one form into another by varying temperature
or pressure is called as Enantiotropic polymorph. Eg. Sulphur.
● One polymorph which is unstable at all temperature & pressure is called as Monotropic
polymorph.
Eg. Glyceryl stearate](https://image.slidesharecdn.com/m-230817053522-073823f7/85/formulation-Development-10-320.jpg)



![1.HYDROLYSIS
● If breaking of molecule is due to reaction with water, then it is
called Hydrolysis.[5]
● It is a step in degradation of substance. Drugs such as esters,
amides and lactams undergo hydrolysis.
● Follows second order kinetics.](https://image.slidesharecdn.com/m-230817053522-073823f7/85/formulation-Development-14-320.jpg)