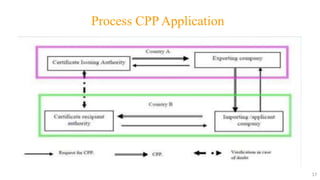
The document provides information about Certificates of Pharmaceutical Product (COPP):
- A COPP is issued by national health authorities in the exporting country upon request from authorities or suppliers in the importing country. It certifies that a pharmaceutical product complies with Good Manufacturing Practices and is approved for sale in the exporting country.
- The COPP allows registration and marketing of the product in the importing country and is necessary for registration renewal. It helps authorities in importing countries assess product quality for import/registration.
- The application process involves submitting documents like manufacturing licenses and quality manuals. Facilities undergo inspection to verify compliance with WHO GMP guidelines. Certifications are issued for approved products and may expire after 24