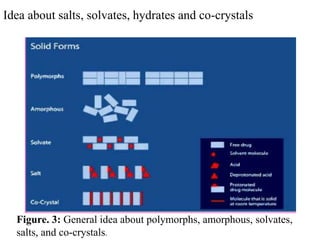
The document discusses preformulation studies that are conducted to characterize the physical, chemical, and mechanical properties of new drug substances. It covers topics like solubility, permeability, polymorphism, hygroscopicity, particle size, and powder flow properties. The objectives of preformulation are to develop stable, safe, and effective dosage forms and generate useful information for formulating an optimal drug delivery system. It also discusses various analytical methods used to characterize solid forms during preformulation.