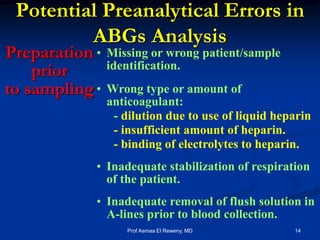
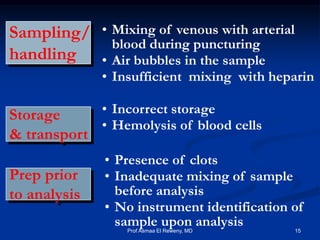
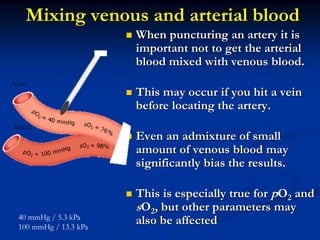
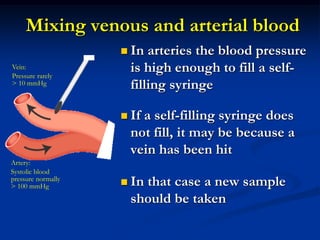
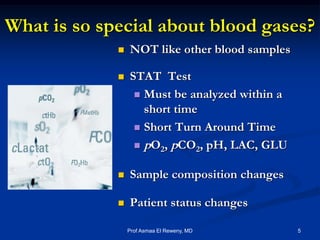
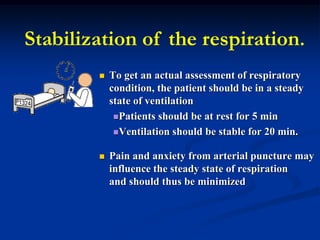
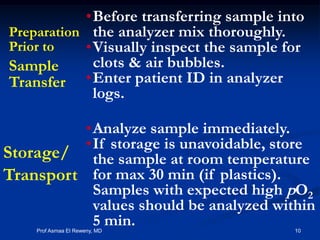
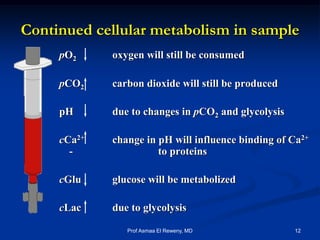
This document discusses preanalytics for arterial blood gas analysis. It emphasizes that the preanalytical phase is the weakest link and stresses the importance of proper sample collection, handling, and transport to ensure accurate results. Potential errors are outlined, including issues with patient stabilization, anticoagulant use, air bubbles, hemolysis, and inadequate mixing or removal of flush solutions prior to sampling. Proper protocols are recommended to minimize preanalytical errors and provide clinically useful results for arterial blood gas analysis.




![Slowing down the metabolism
Blood gas samples in glass samplers
could be cooled: storing the sample
at lower temperature (0-4 °C) will
slow down metabolism by at least a
factor of 10 [NCCLS].
Cool samples in an ice slurry or
other suitable coolant.
Never store the samples directly on
ice as this causes hemolysis of blood
cells.
NCCLS Document C27-A; Blood Gas Pre-Analytical Considerations: Specimen Collection, Calibrations and Controls; Approved Guideline
25 C
0-4 C
pO2
Time
13Prof Asmaa El Reweny, MD](https://image.slidesharecdn.com/preanalyticalerrorsabgs-170309053315/85/Preanalytical-errors-ab-gs-13-320.jpg)