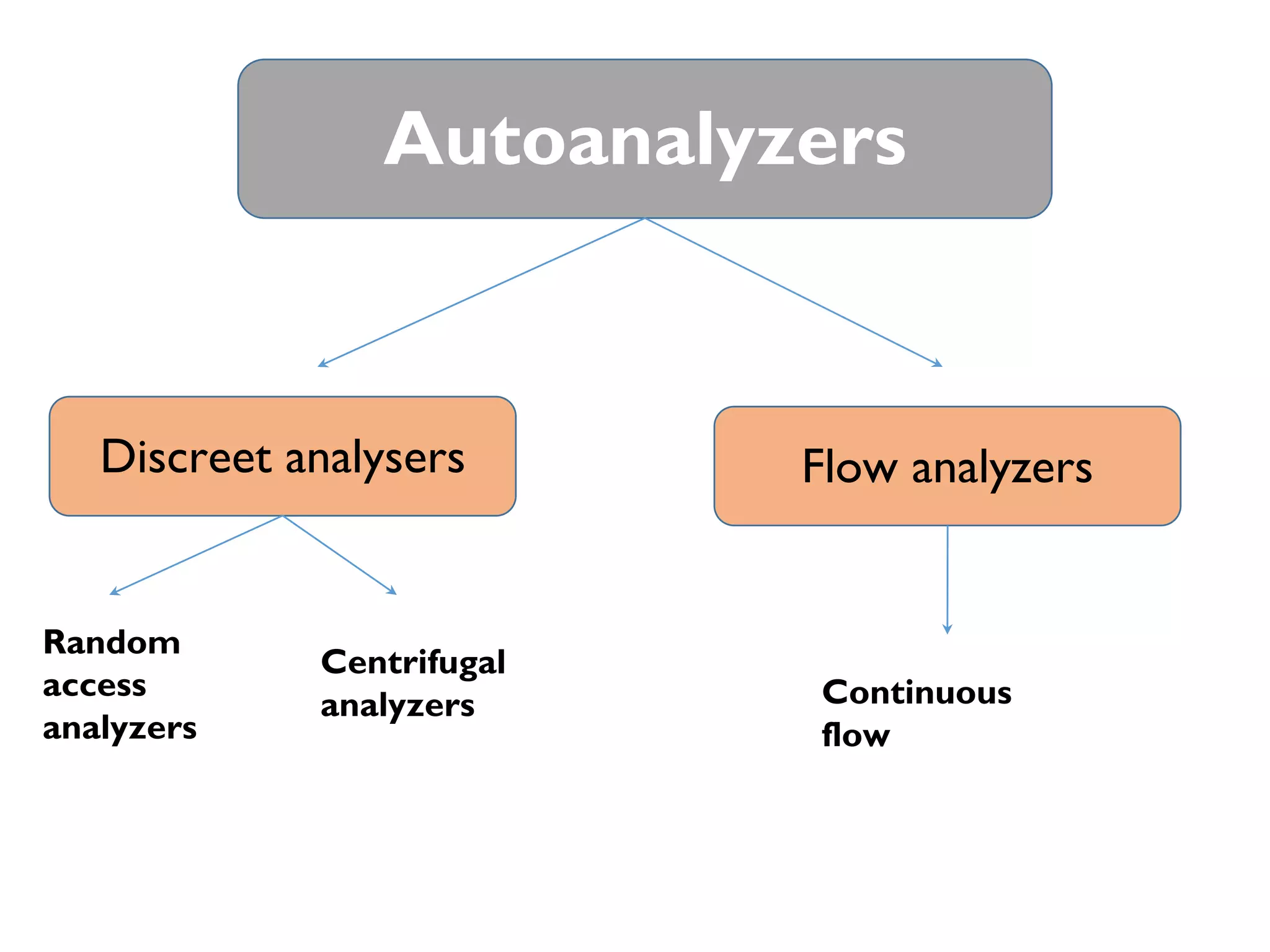
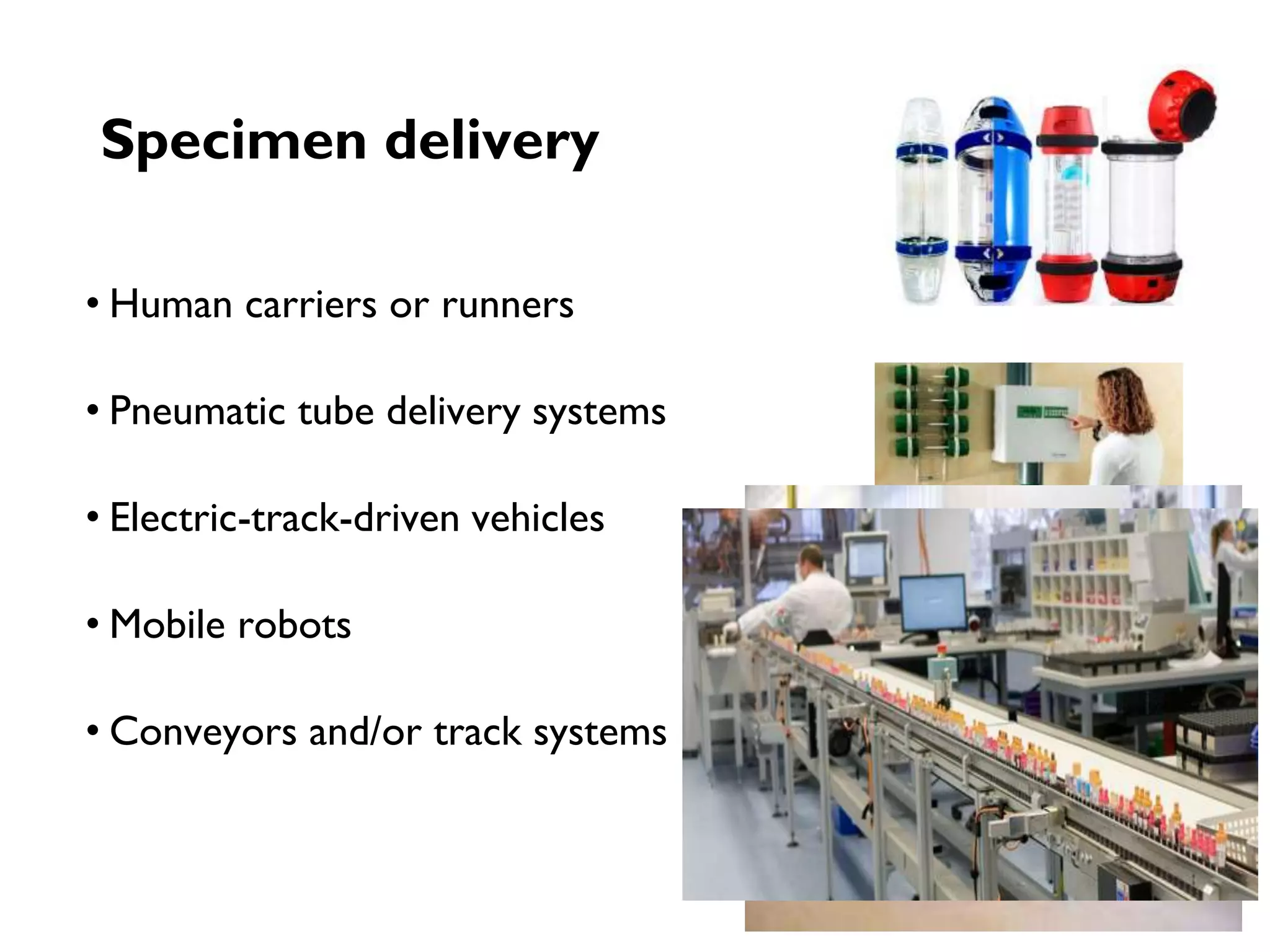
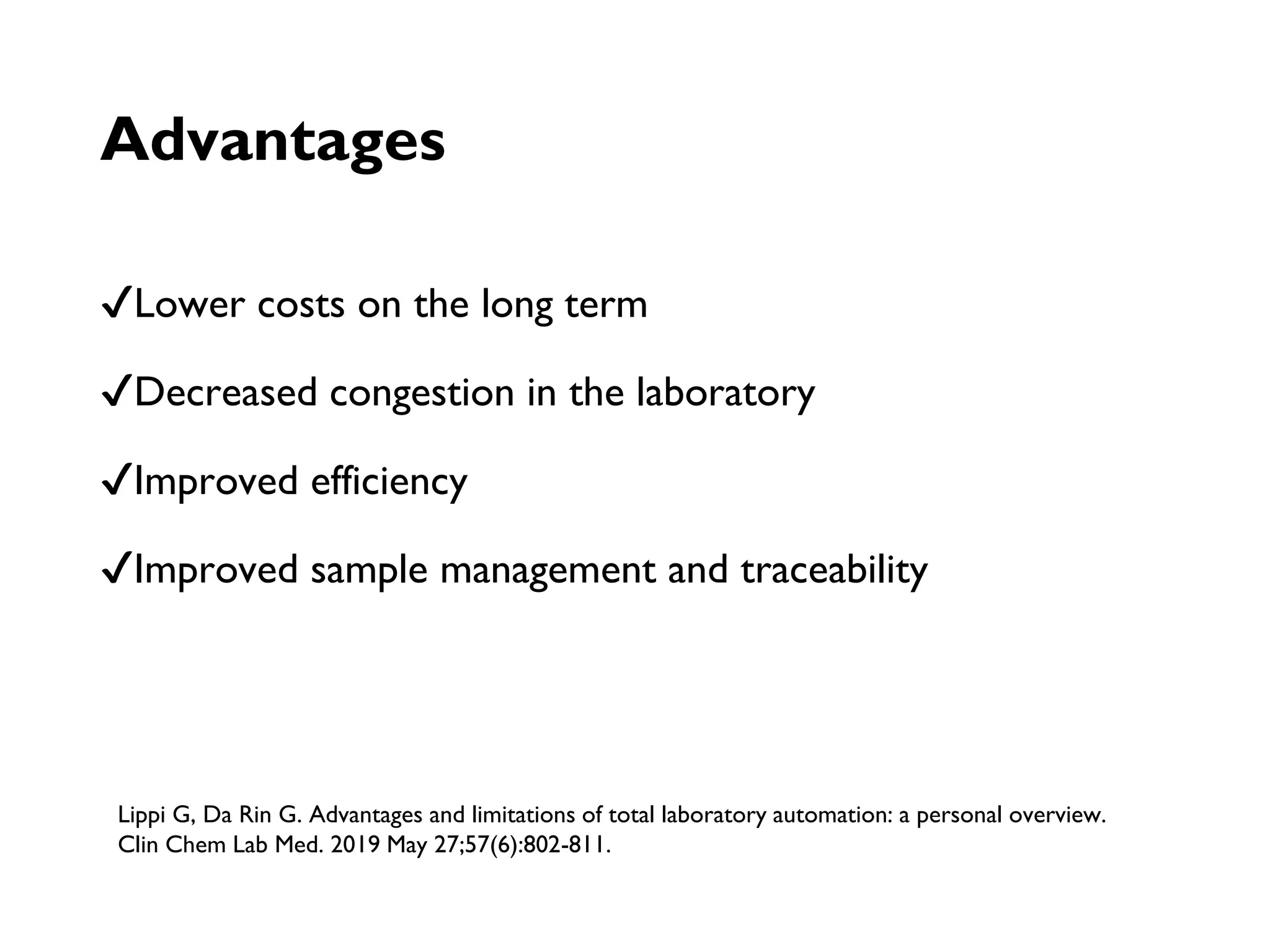
Automation in clinical laboratories aims to minimize manual processes and variations. It divides work into pre-analytic, analytic and post-analytic phases, with the analytic phase being the most automated. Different types of automated analyzers include continuous flow, centrifugal, discrete and random access analyzers. Total laboratory automation integrates many analyzers and physically connects processes to improve efficiency, quality and reduce costs over time despite higher initial costs. While automation decreases errors and biological risk, it can increase infrastructure needs and downtime risks.