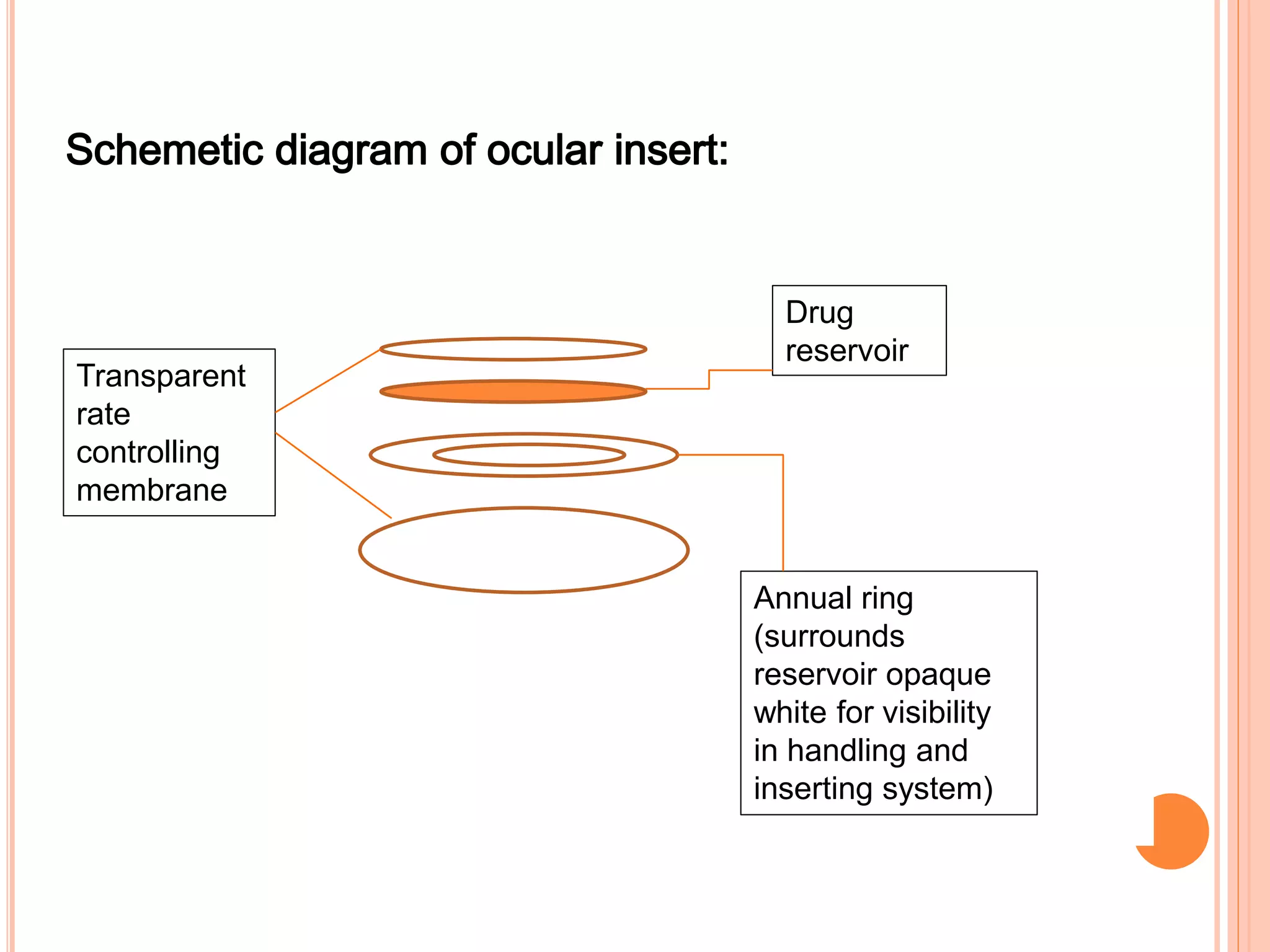
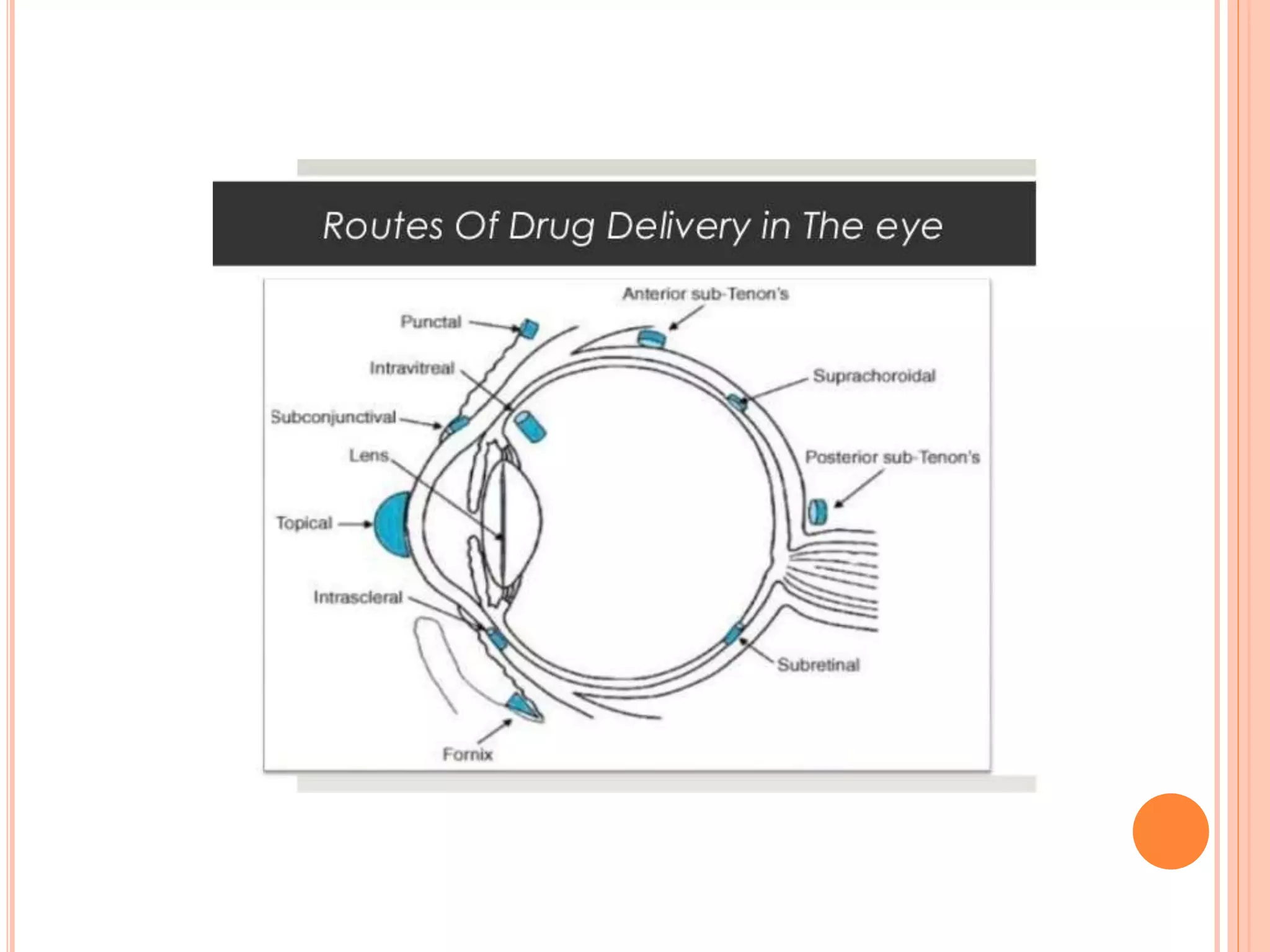
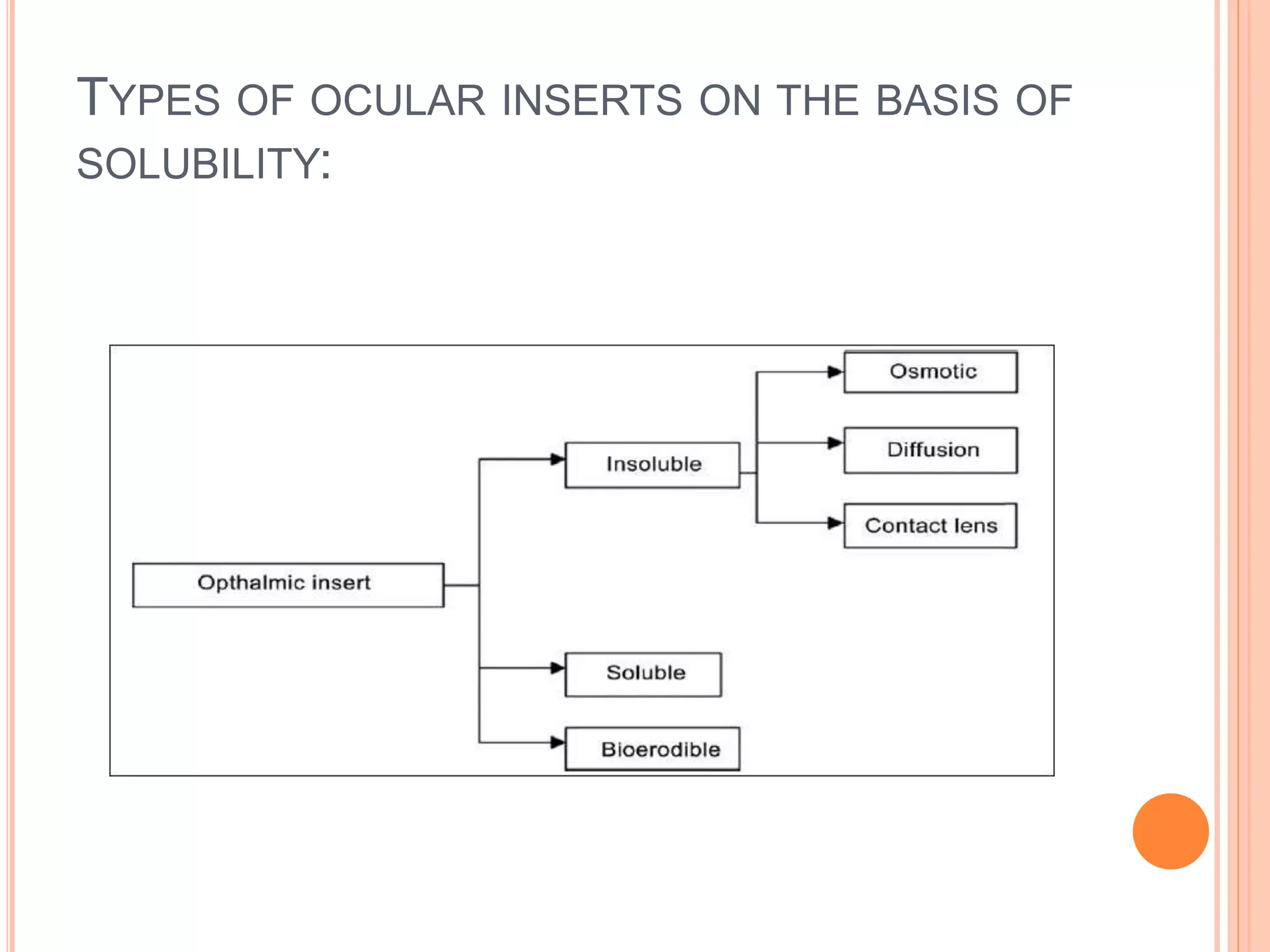
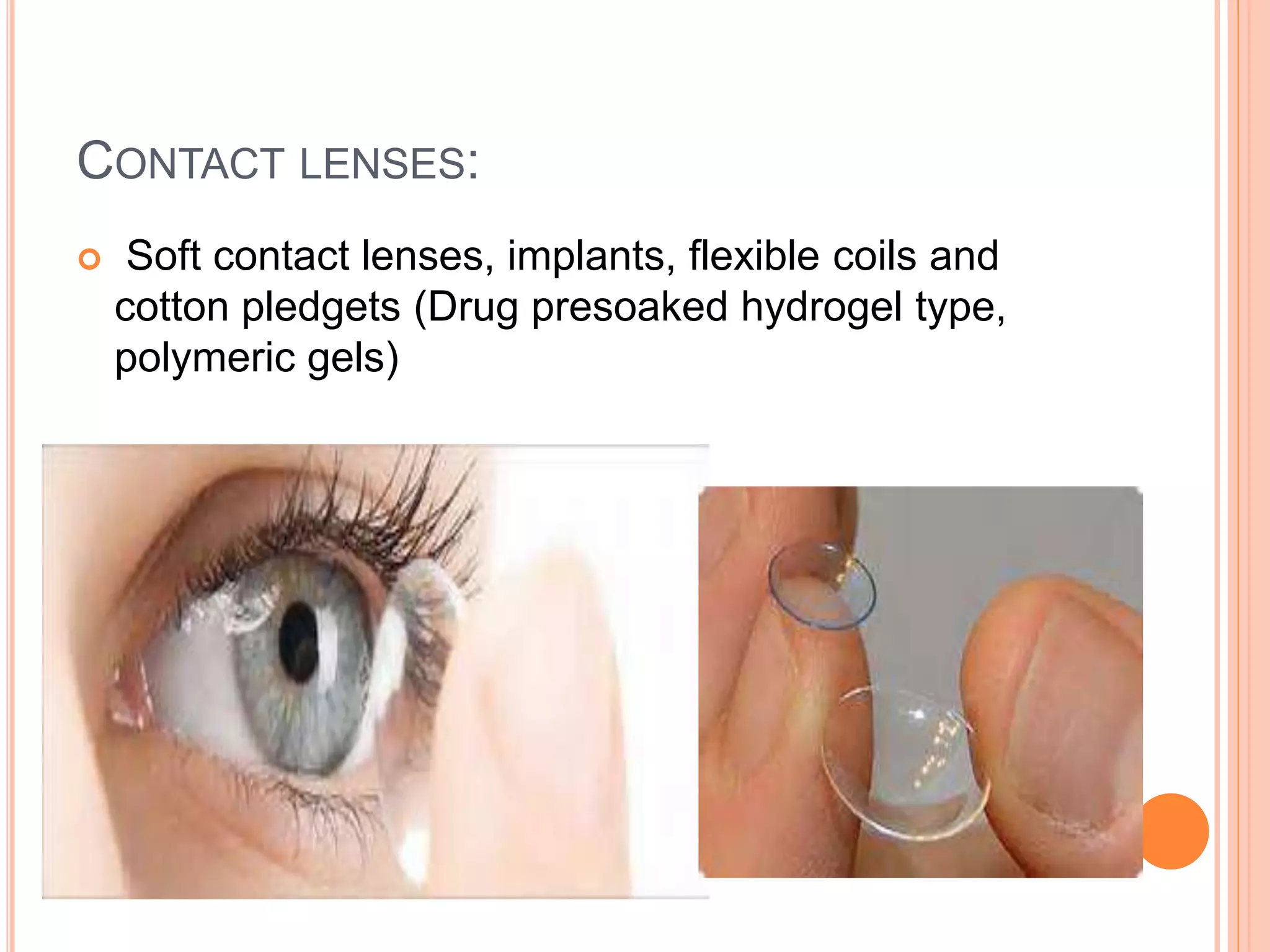
The document discusses ocular inserts, which are thin, solid or semi-solid drug-impregnated devices placed in the eye to provide prolonged drug delivery. It defines ocular inserts and describes different types including soluble, insoluble, and erodible inserts. Applications include treatments for glaucoma, infections, and inflammation. Advantages are prolonged contact time and drug release, while disadvantages include potential loss or irritation. The document outlines manufacturing methods and innovations in ocular insert technologies and drug delivery to the eye.