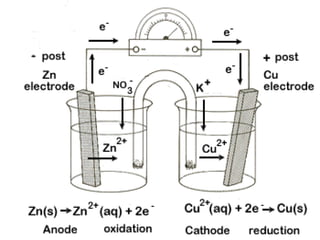
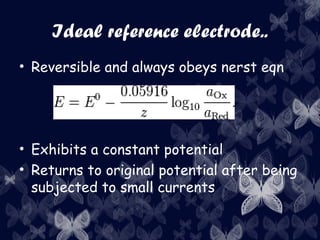
Electroanalytical chemistry techniques can determine the concentration, stoichiometry, and activity of chemical species using electrical properties. Potentiometry specifically measures electrode potentials to analyze ion concentrations. Reference electrodes have a constant potential and are unaffected by the analyte composition, allowing measurement of a cell potential relative to the reference. Ion-selective electrodes convert specific ion activity into a measurable potential via selective membranes based on ion exchange, crystallization, or complexation.