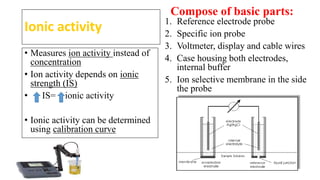
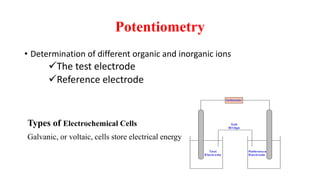
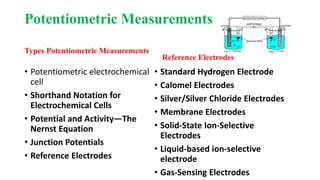
This document discusses various electrochemical methods and their applications. It covers topics like potentiometry, types of electrochemical cells, reference electrodes, quantitative applications in clinical and environmental analysis, and potentiometric titrations. Ion selective electrodes are explained in detail, including how they work, types of analytes and electrodes, maintenance, and applications in various fields like analytical chemistry, clinical diagnostics, and environmental monitoring. Different types of voltammetry techniques are also introduced.



![Introduction to ISE
• It’s a galvanic cell which is based on movement of ions without oxidation or reduction
reaction take place Ecell = EISE – Eref
• Also known as indicator electrodes.
• Used for direct potentiometric measurement.
We are concern here with “ION” not a protein.
ISE: Ion for +/- ions and selective : for specific ion by membrane used in it and
utilize electrode for measuring the potential by voltmeter.
Specificity and sensitivity = ISE = separation and detection
Nernst equation is then drive for pH or [H+] electrical voltage is related to ion
concentration](https://image.slidesharecdn.com/electrochemicalmethods-240201174305-cce157b1/85/Methods-in-Electrochemical-in-chemistry-6-320.jpg)
![ In 1909, Danish chemist work on “pH meter” with principle
of potentiometric
[H+] in (mmol/L) in a solution led to discover conventional
pH scale (1 to 14)
Combine electrode been design since 1940s](https://image.slidesharecdn.com/electrochemicalmethods-240201174305-cce157b1/85/Methods-in-Electrochemical-in-chemistry-7-320.jpg)