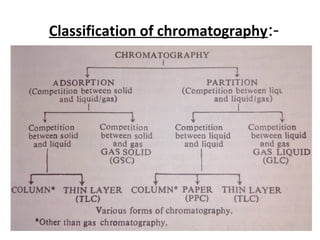
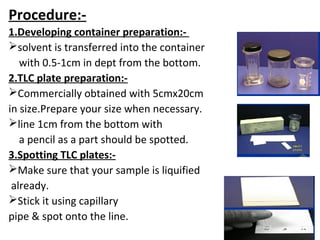
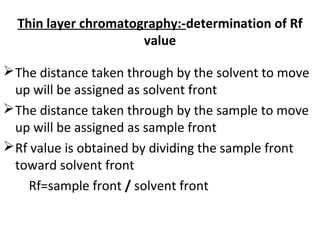
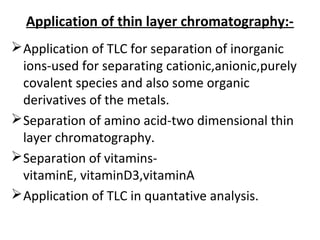
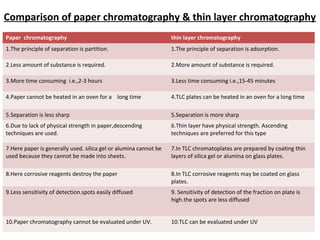
This document provides an overview of chromatography techniques. It discusses the definition and history of chromatography, and describes several types including paper chromatography, thin layer chromatography, column chromatography, gas chromatography, and high performance liquid chromatography. Specific details are provided on the principles, procedures, applications, advantages and disadvantages of paper chromatography and thin layer chromatography. Key differences between these two techniques are also compared.