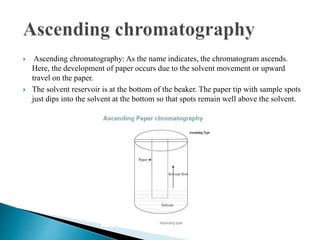
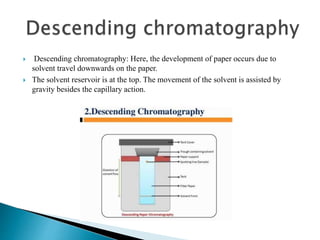
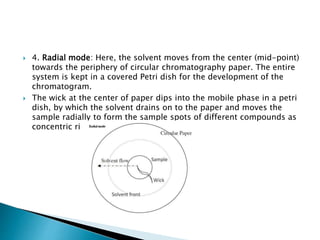
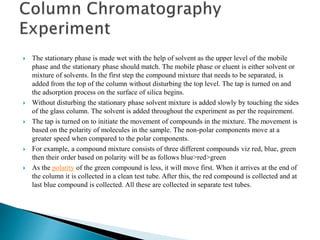
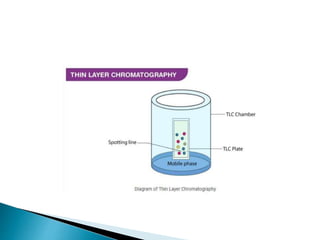
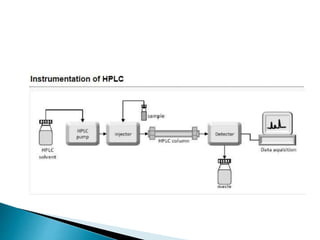
Chromatography is a technique used to separate mixtures based on how their constituents partition between a stationary and mobile phase. In paper chromatography, the stationary phase is held in the pores of filter paper while the mobile phase moves over the paper. Different compounds travel at different speeds depending on how they partition between the water in the stationary phase and the mobile solvent, allowing separation. Column chromatography uses a column filled with a stationary phase like silica gel and a mobile phase solvent that carries compounds through the column at different rates based on their interactions with the stationary phase. Thin layer chromatography plates have a thin stationary phase coating and compounds are separated as they move up the plate with the mobile phase solvent, appearing as separated spots. These techniques are used