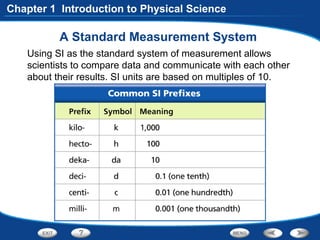
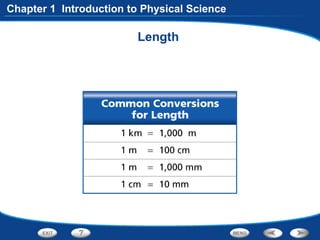
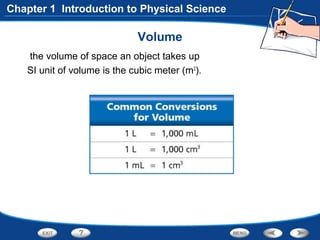
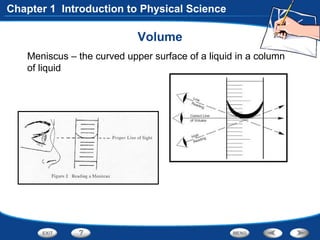
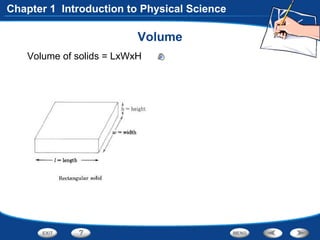
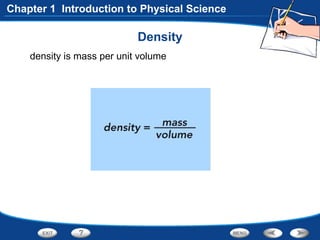
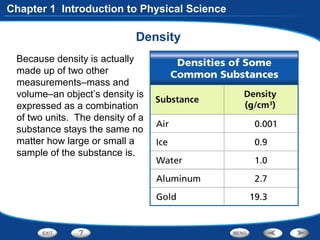
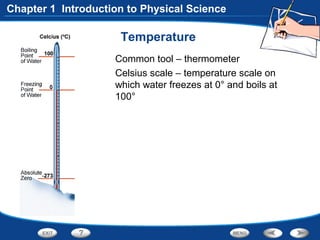
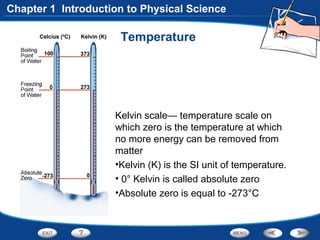
The document discusses the International System of Units (SI) as the standard measurement system used internationally. It describes the basic SI units for common physical quantities like length, mass, time, temperature, and others. For example, the basic unit of length is the meter (m) and the basic unit of mass is the kilogram (kg). Using a standard international system of units allows scientists to easily compare and share data.