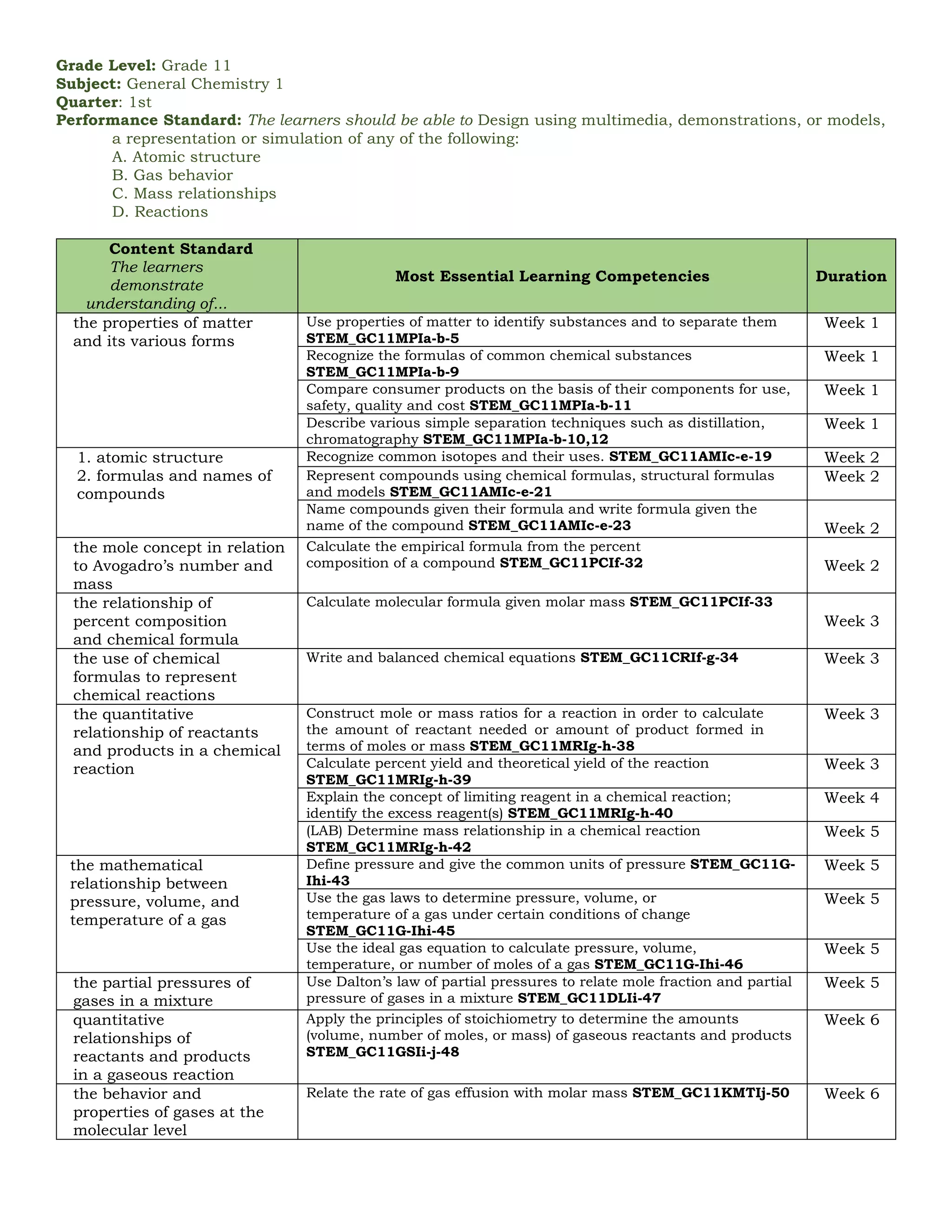
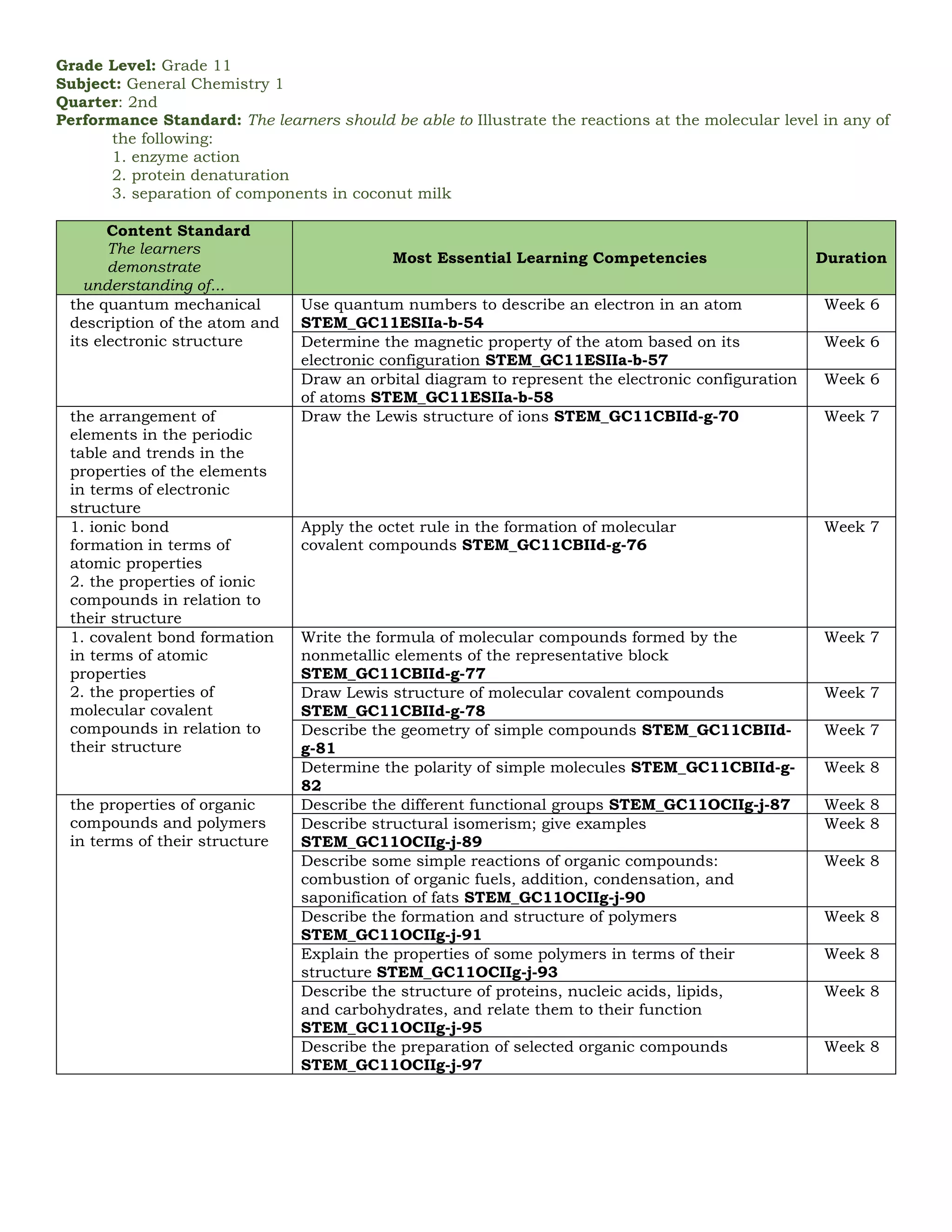
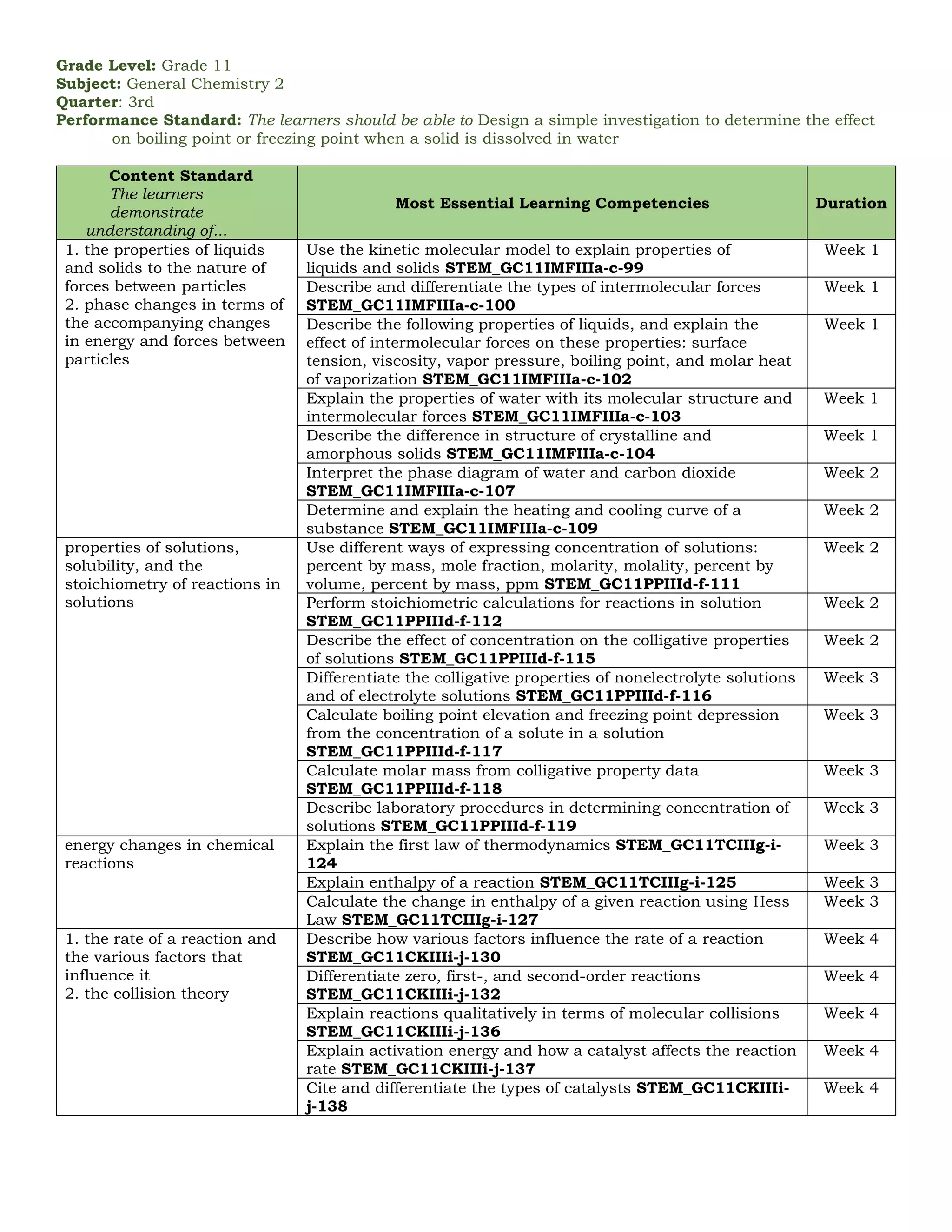
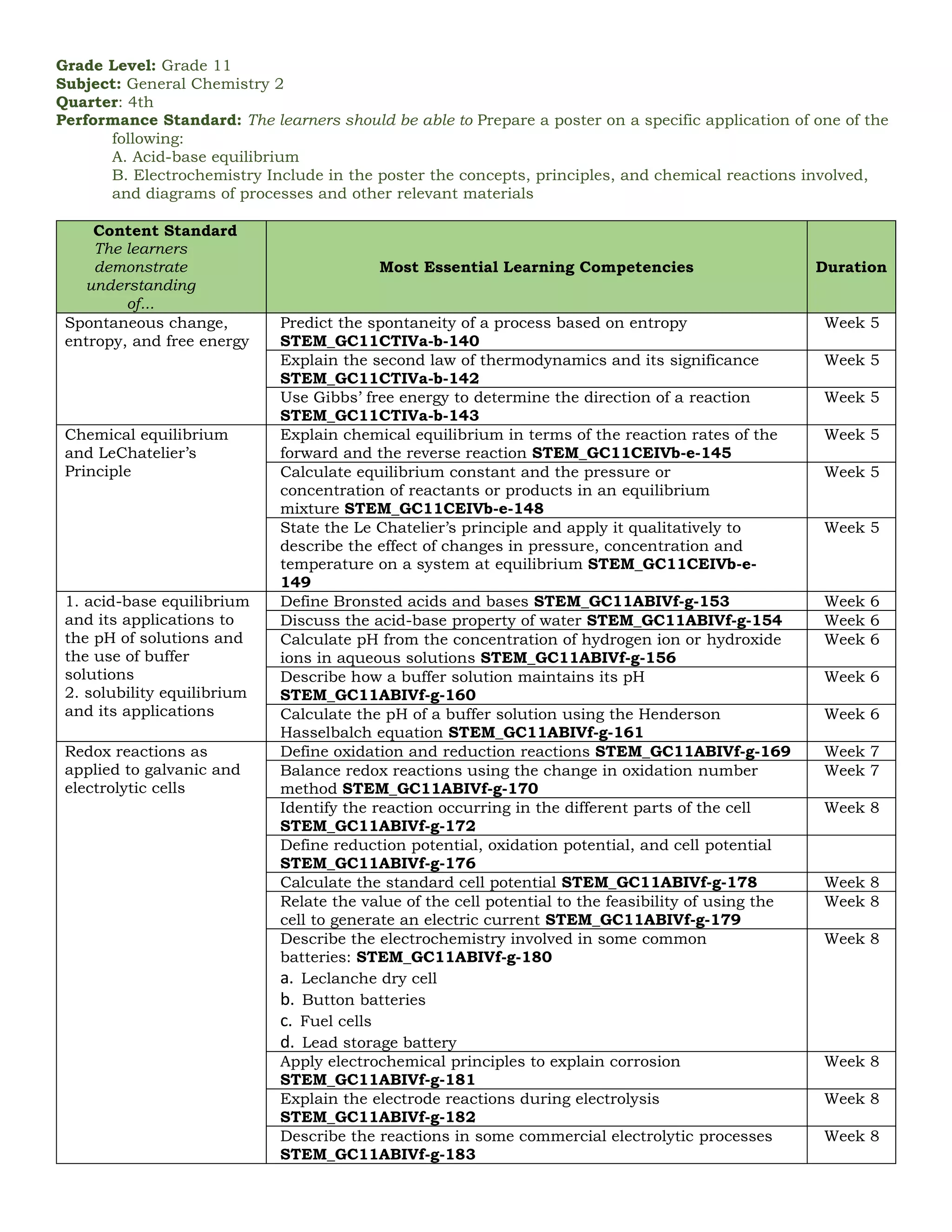
The document outlines the essential learning competencies for senior high school (STEM) students in general chemistry across various quarters and topics, including atomic structure, chemical reactions, gas behavior, and thermodynamics. It specifies performance standards, detailed competencies for each week, and the skills students should acquire, such as recognizing chemical formulas, understanding the mole concept, and applications of acid-base equilibrium and electrochemistry. The content supports students in developing a strong foundational knowledge and practical skills necessary for further studies in chemistry.