This document provides an overview of Phase 0 clinical trials, also known as microdosing trials. It discusses:
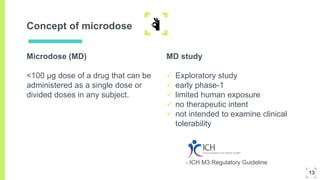
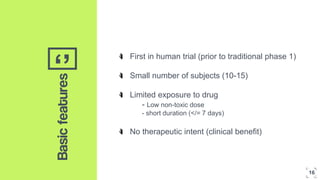
1. The concept of microdosing involves administering very low, subtherapeutic doses of new drug candidates to humans to obtain pharmacokinetic and pharmacodynamic data early in development.
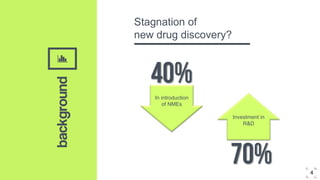
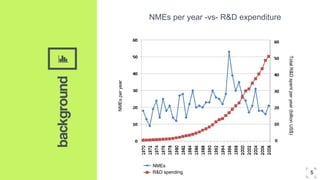
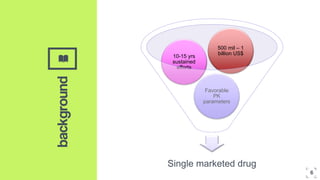
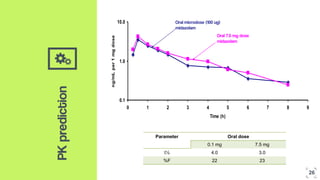
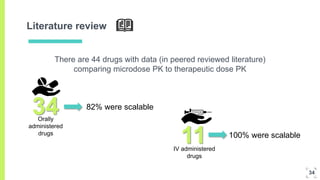
2. Microdosing trials have several goals including predicting human pharmacokinetics better than animal models to facilitate candidate selection and eliminate unsuccessful compounds earlier.
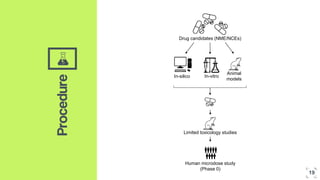
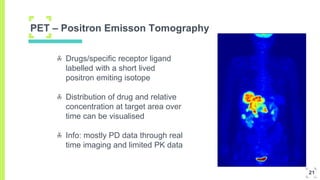
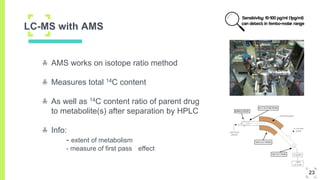
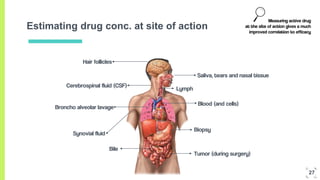
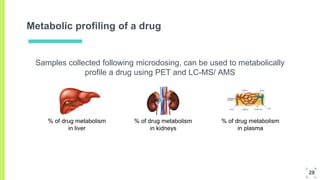
3. The procedure involves collecting and analyzing plasma, urine or biopsy samples using sensitive techniques like PET scans and LC-MS to measure low drug concentrations.
4. Microdosing has potential applications in areas like predicting human pharmacokinetics,