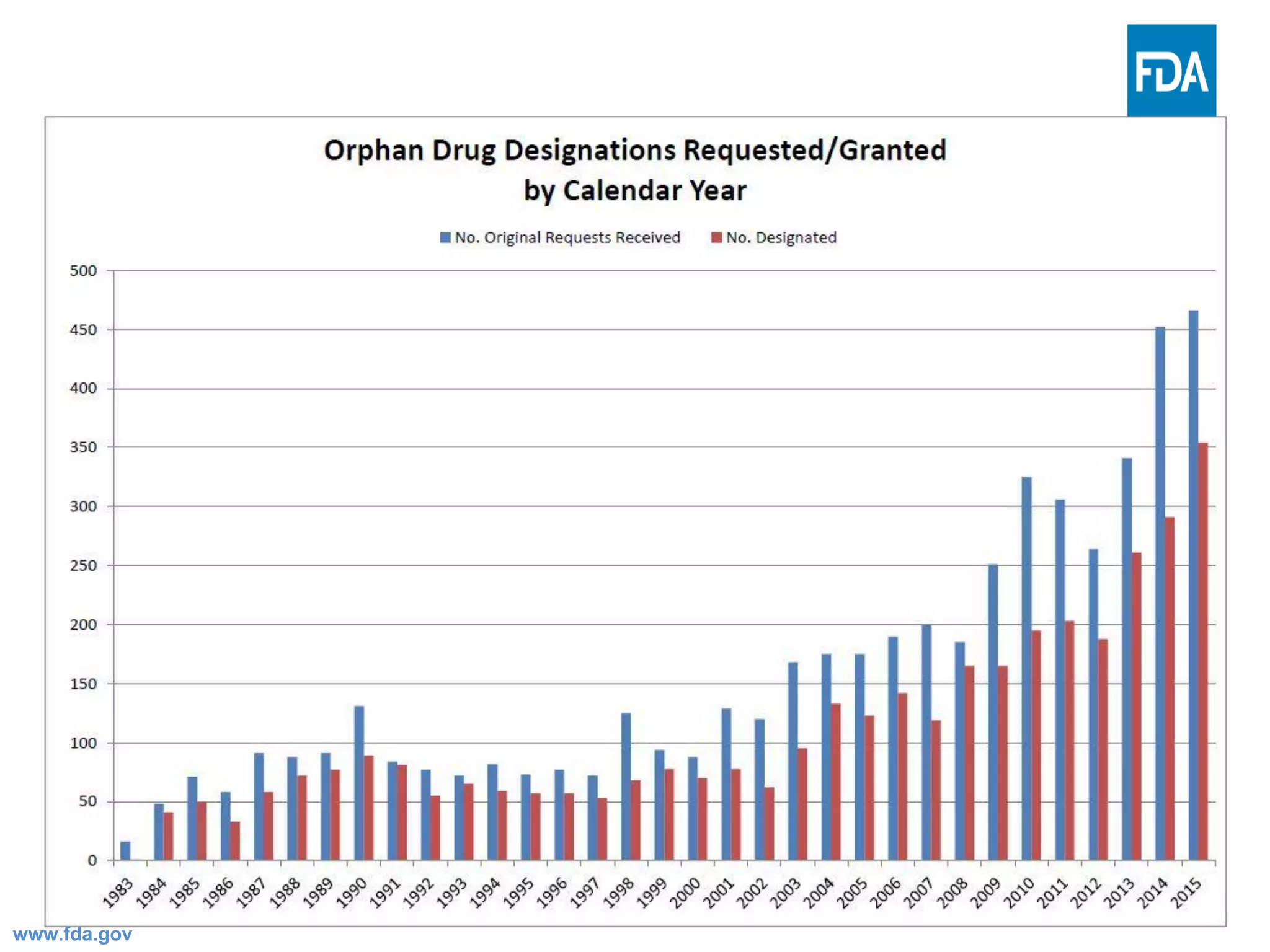
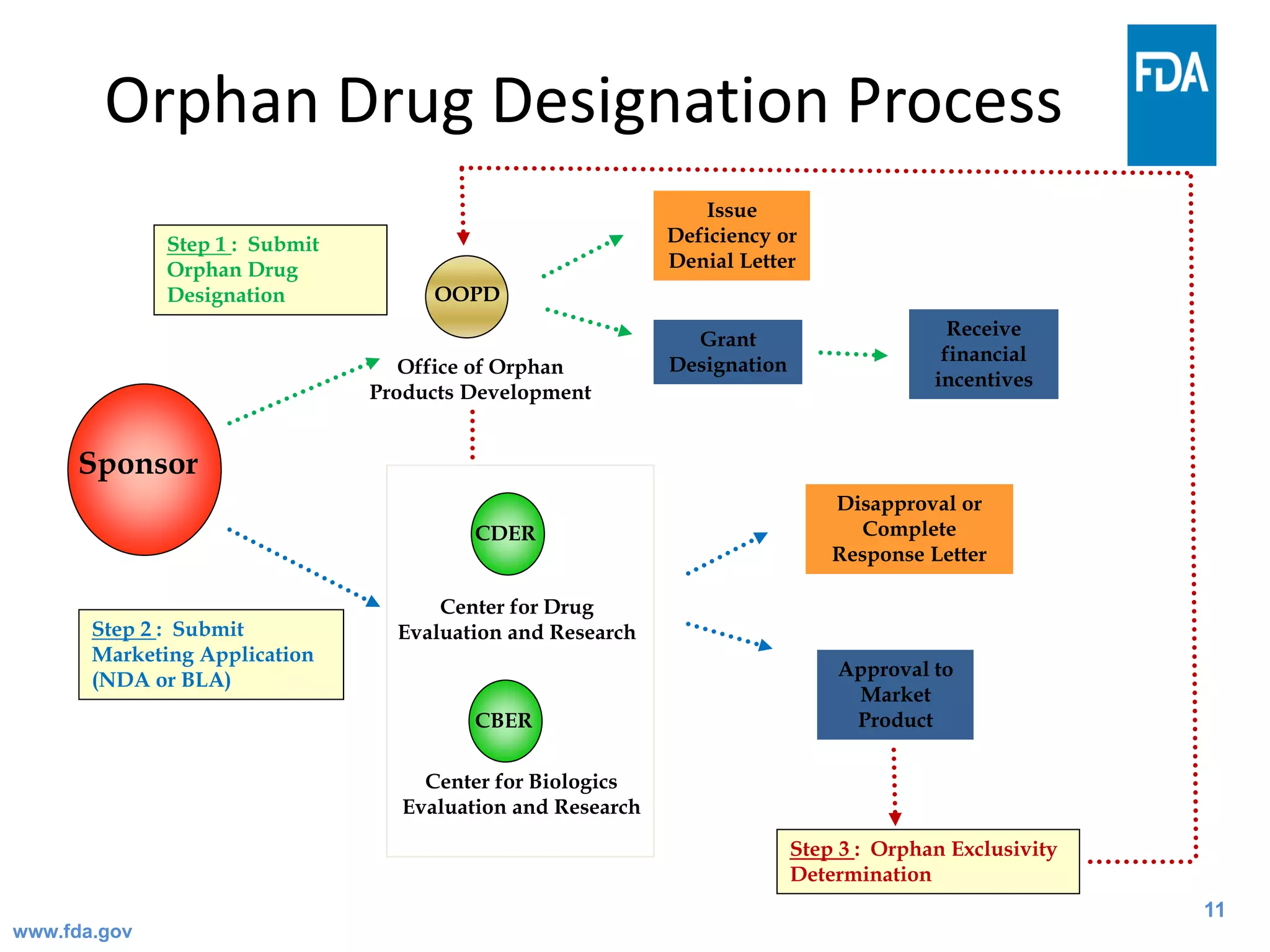
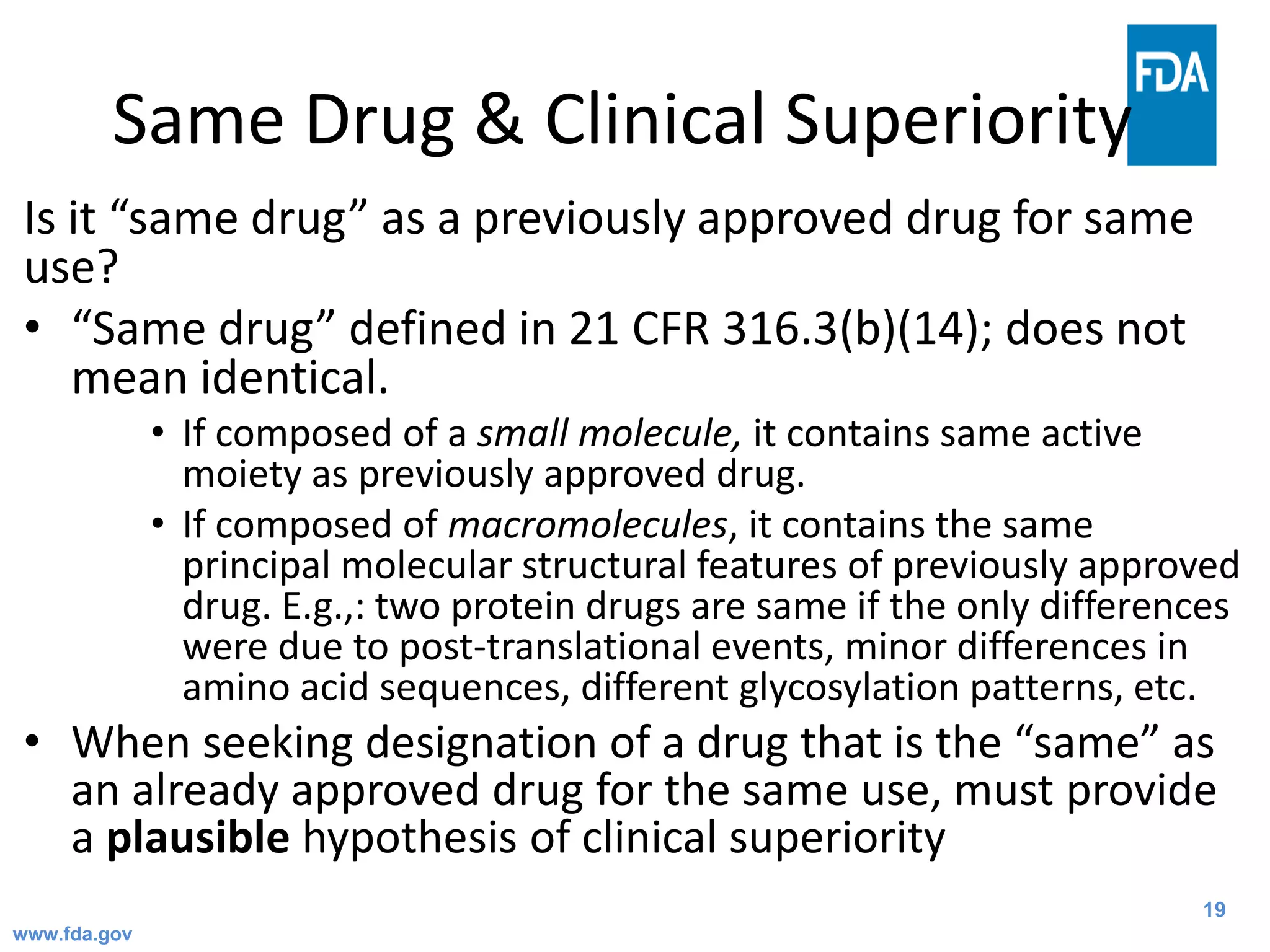
The Office of Orphan Products Development (OOPD) at the FDA promotes the development of treatments for rare diseases and conditions. There are more than 6,800 known rare diseases affecting an estimated 25-30 million Americans. The Orphan Drug Act of 1983 provides financial incentives like tax credits, user fee waivers, and exclusive marketing rights for 7 years to encourage development of treatments for rare diseases. The OOPD oversees programs that grant orphan drug designation, provide funding for clinical trials and natural history studies, and award priority review vouchers for rare pediatric diseases.