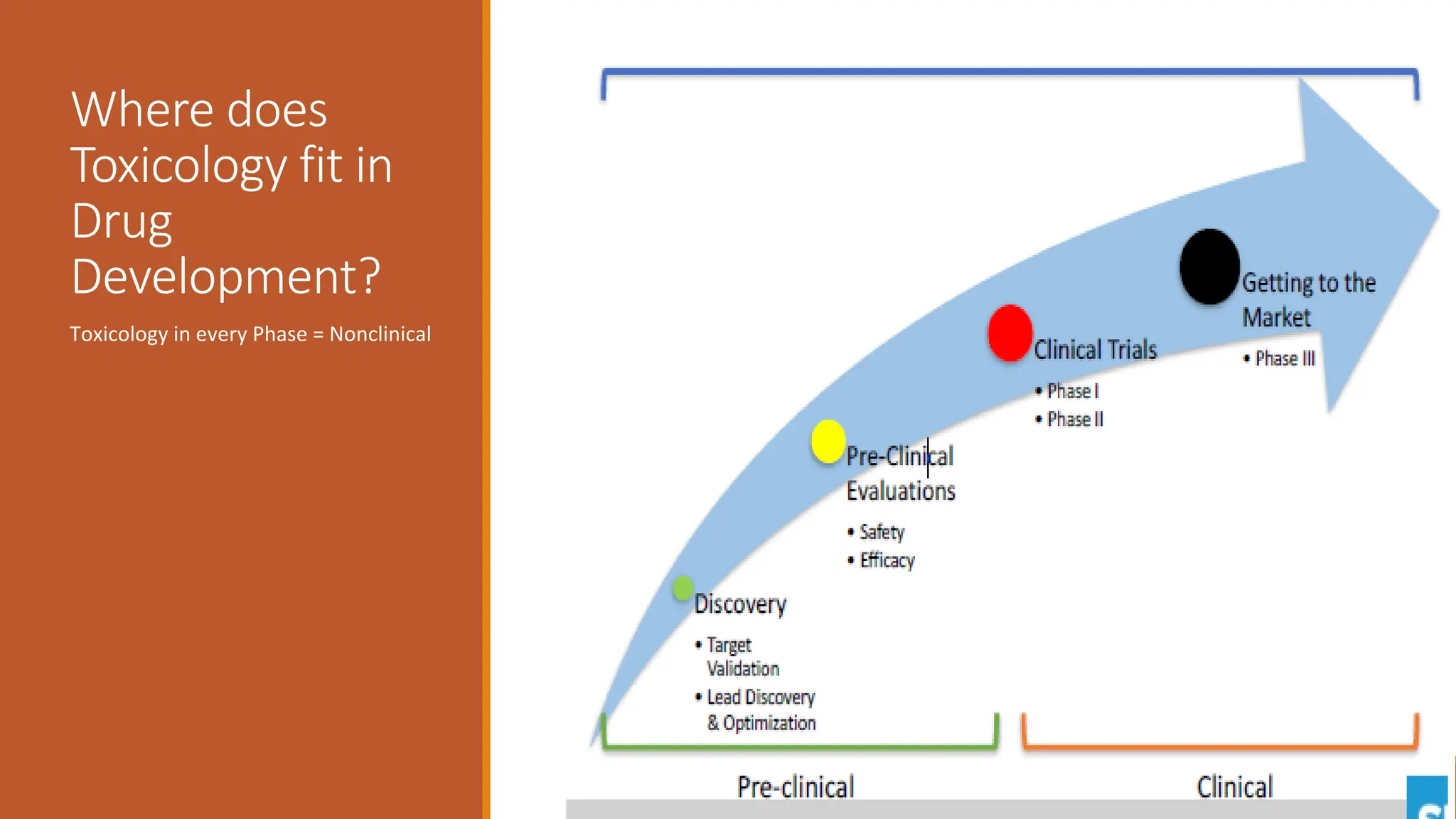
The document discusses the principles and importance of non-clinical toxicity studies in drug development, emphasizing the identification and assessment of hazardous effects caused by toxic agents. It outlines various types of toxicity studies, including single-dose and repeated-dose toxicity, genotoxicity, carcinogenicity, and how these studies inform clinical trial decisions and regulatory submissions. The document also covers methodologies for toxicokinetics, the ethical considerations of using animal models, and how to determine safe first-in-human doses.