The document discusses extrapolating data from preclinical in vitro and in vivo animal studies to humans in clinical trials. It provides information on different types of studies and explains how data from animal models is used to estimate safe starting doses for human subjects. The key points are:
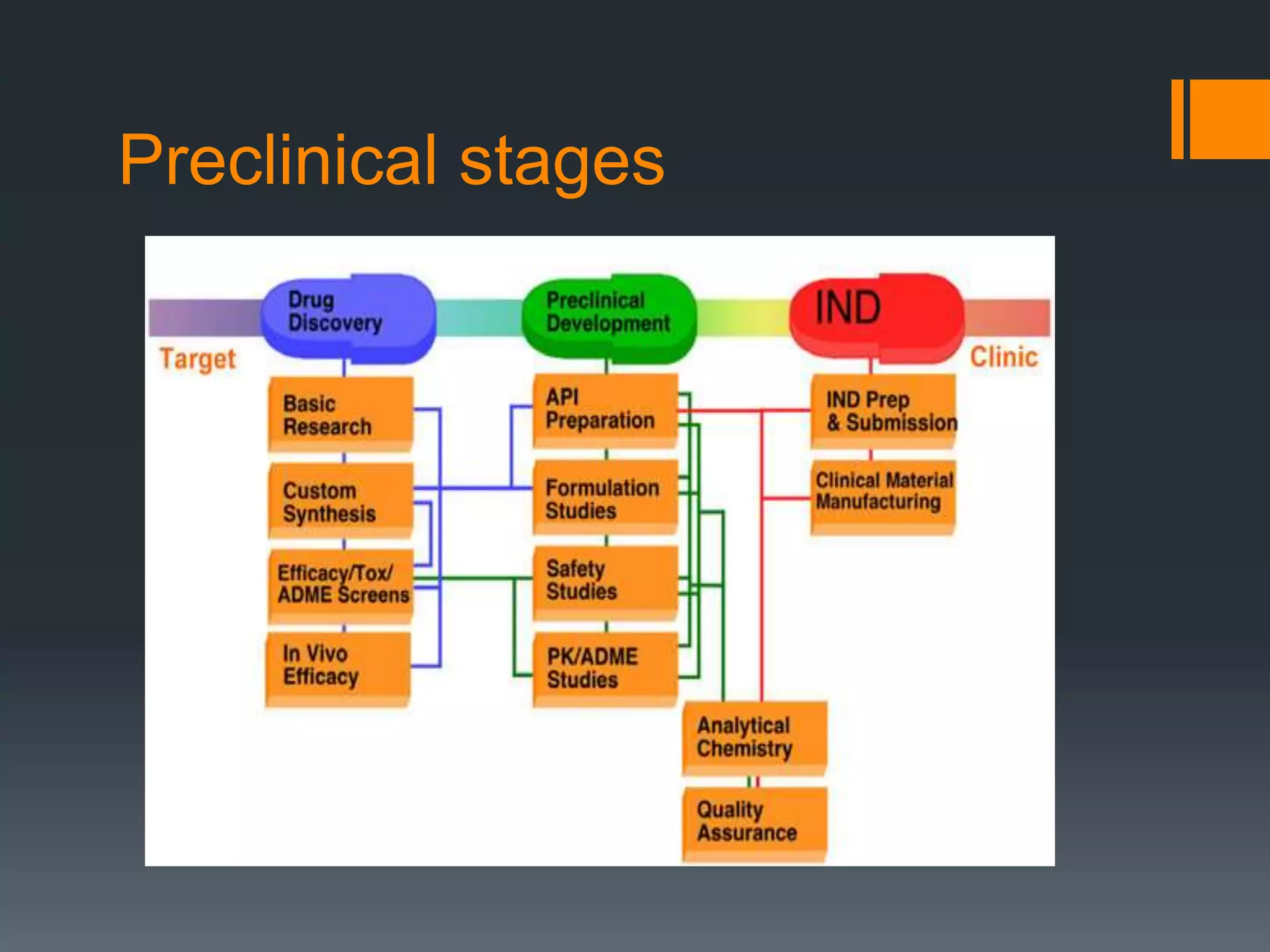
1) Preclinical studies test drugs in animal and cell models before human trials to evaluate toxicity and effects. Data from these studies is extrapolated using mathematical processes to estimate appropriate human doses.
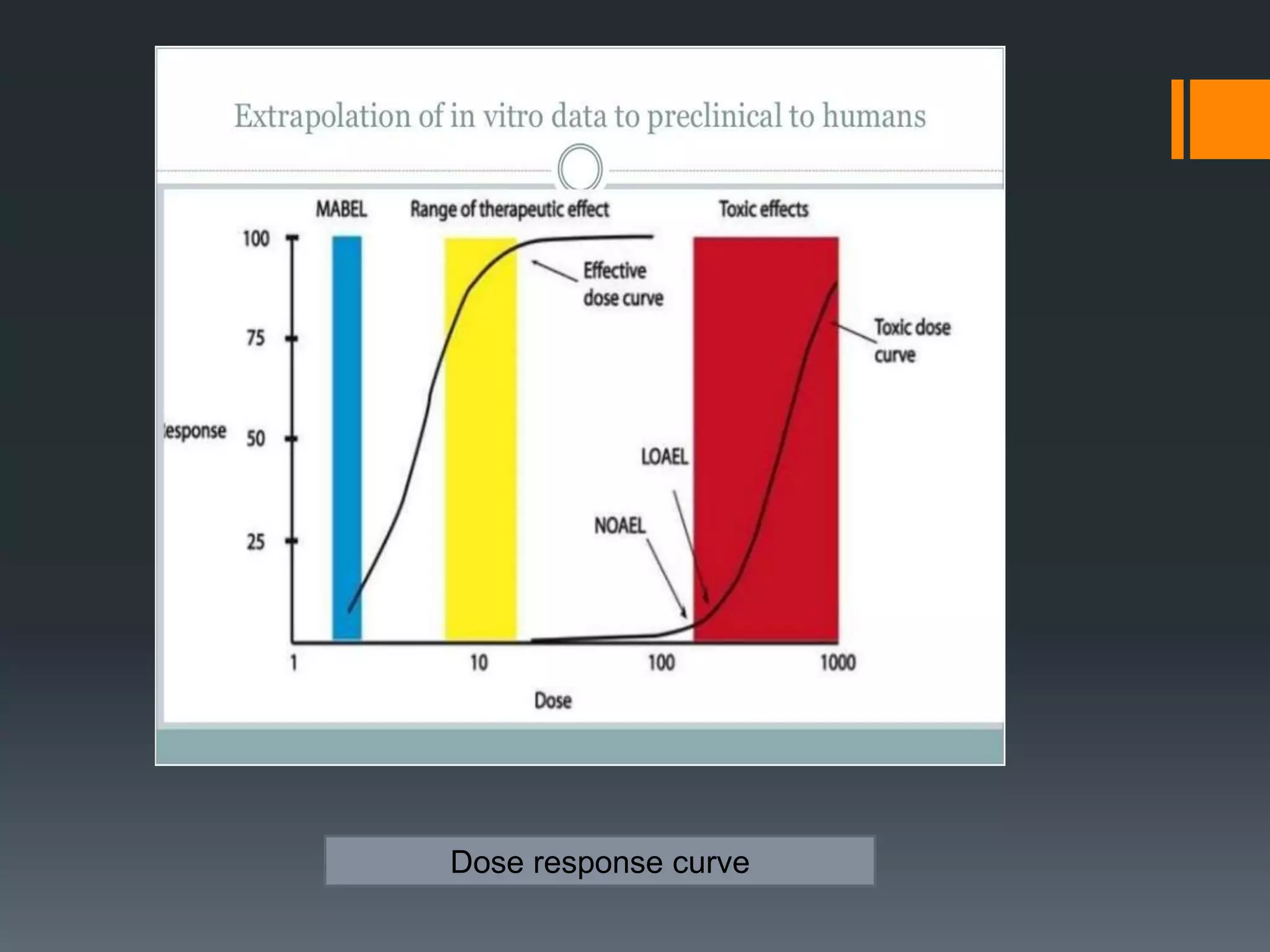
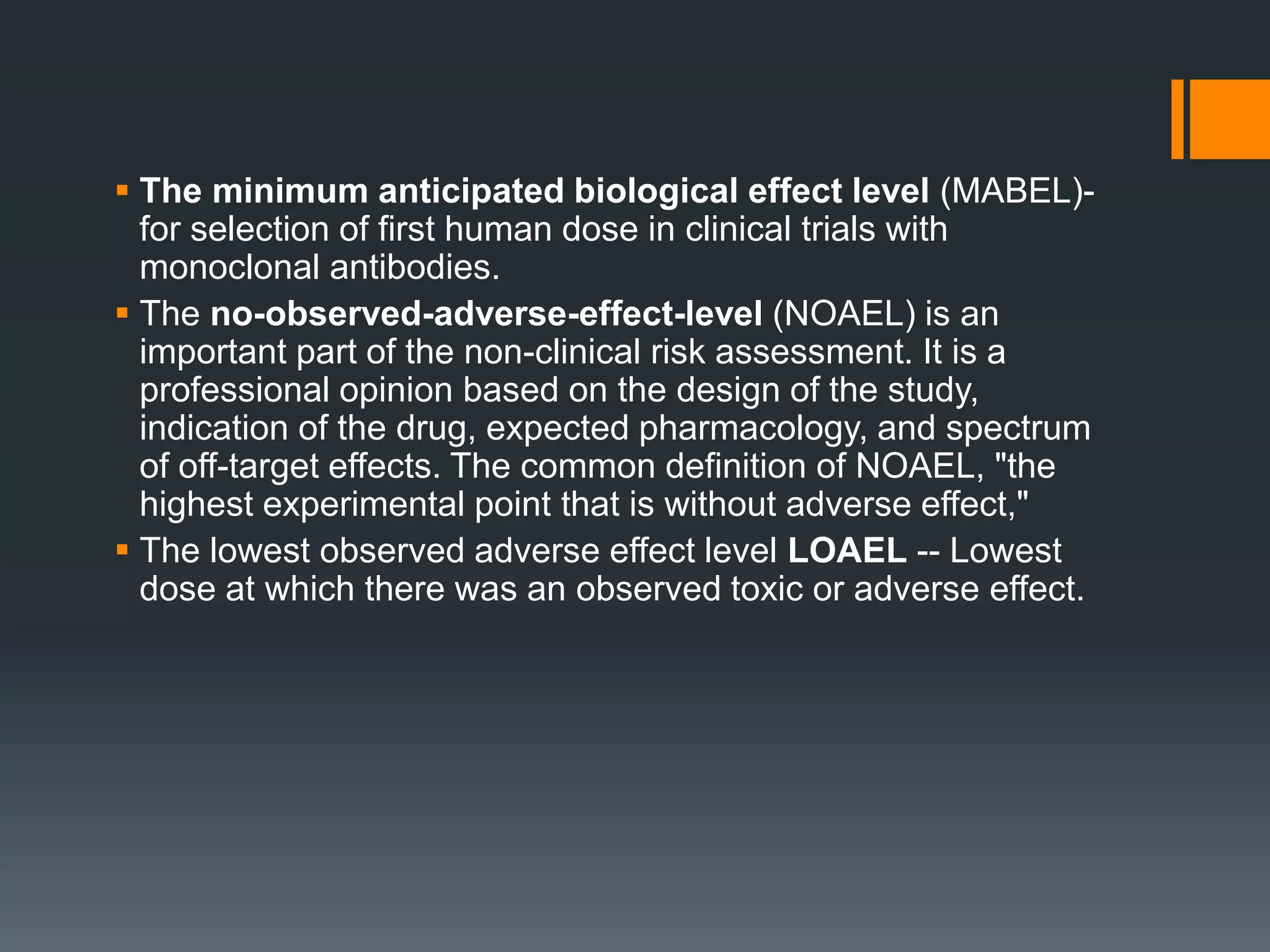
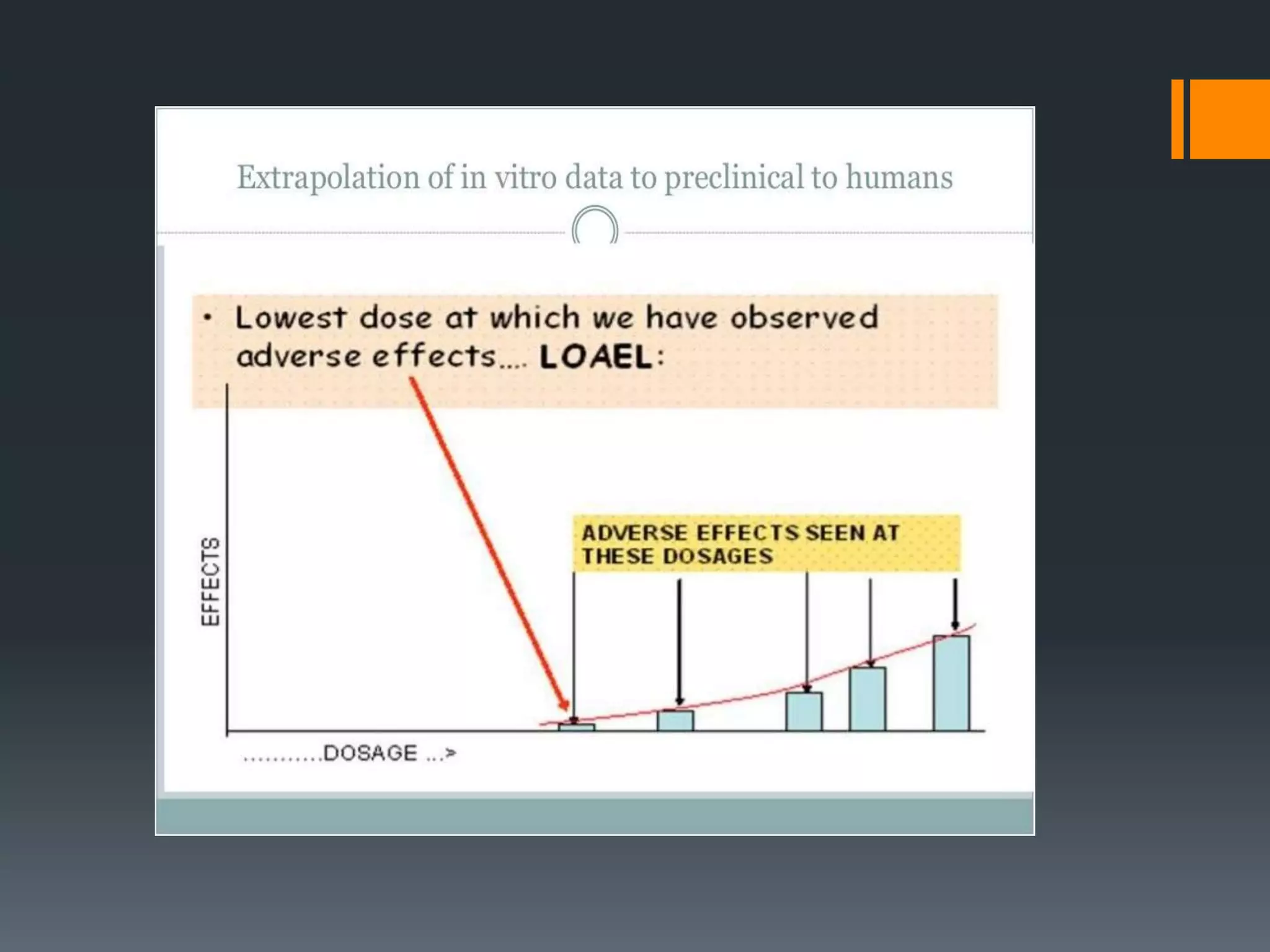
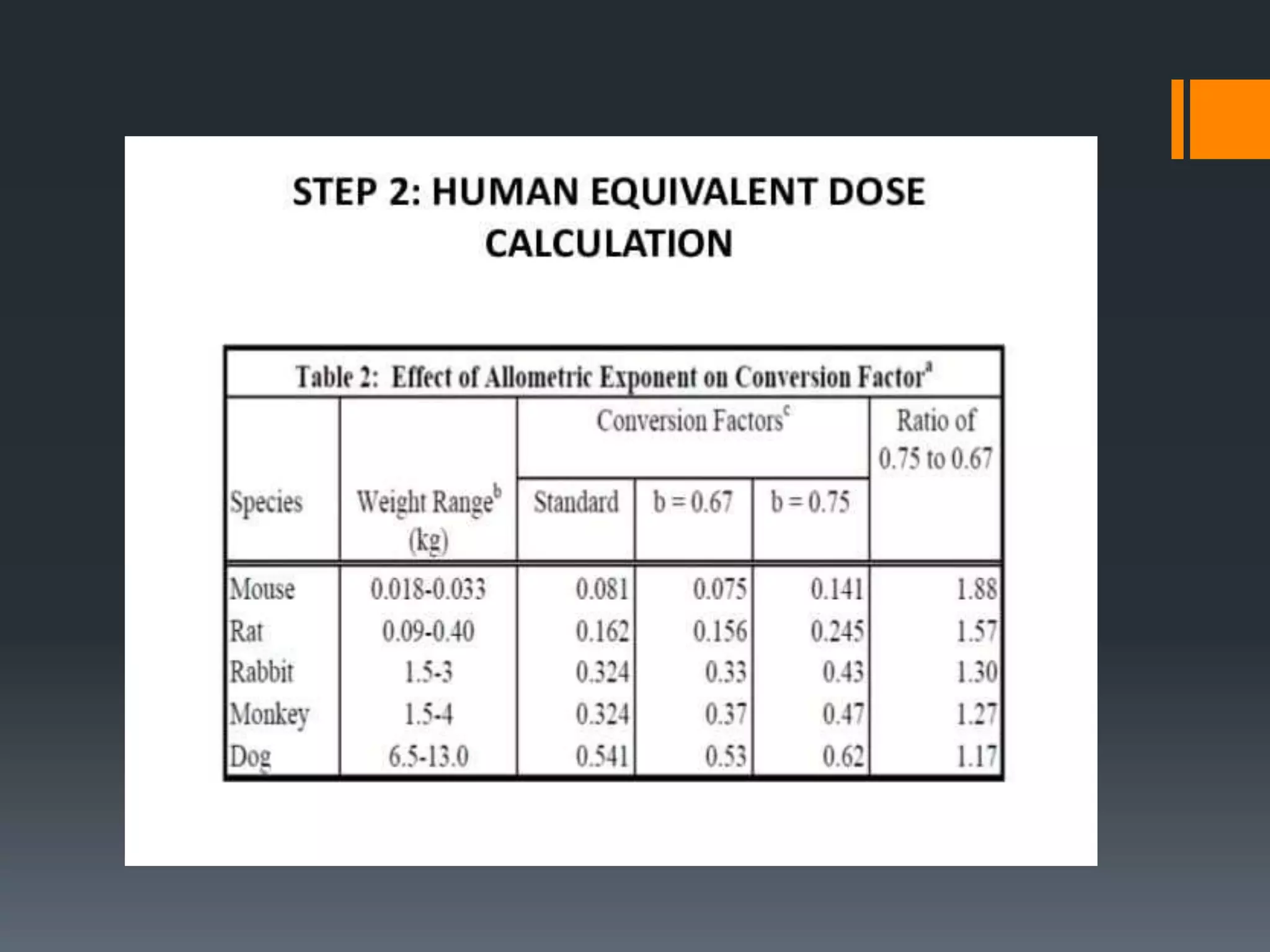
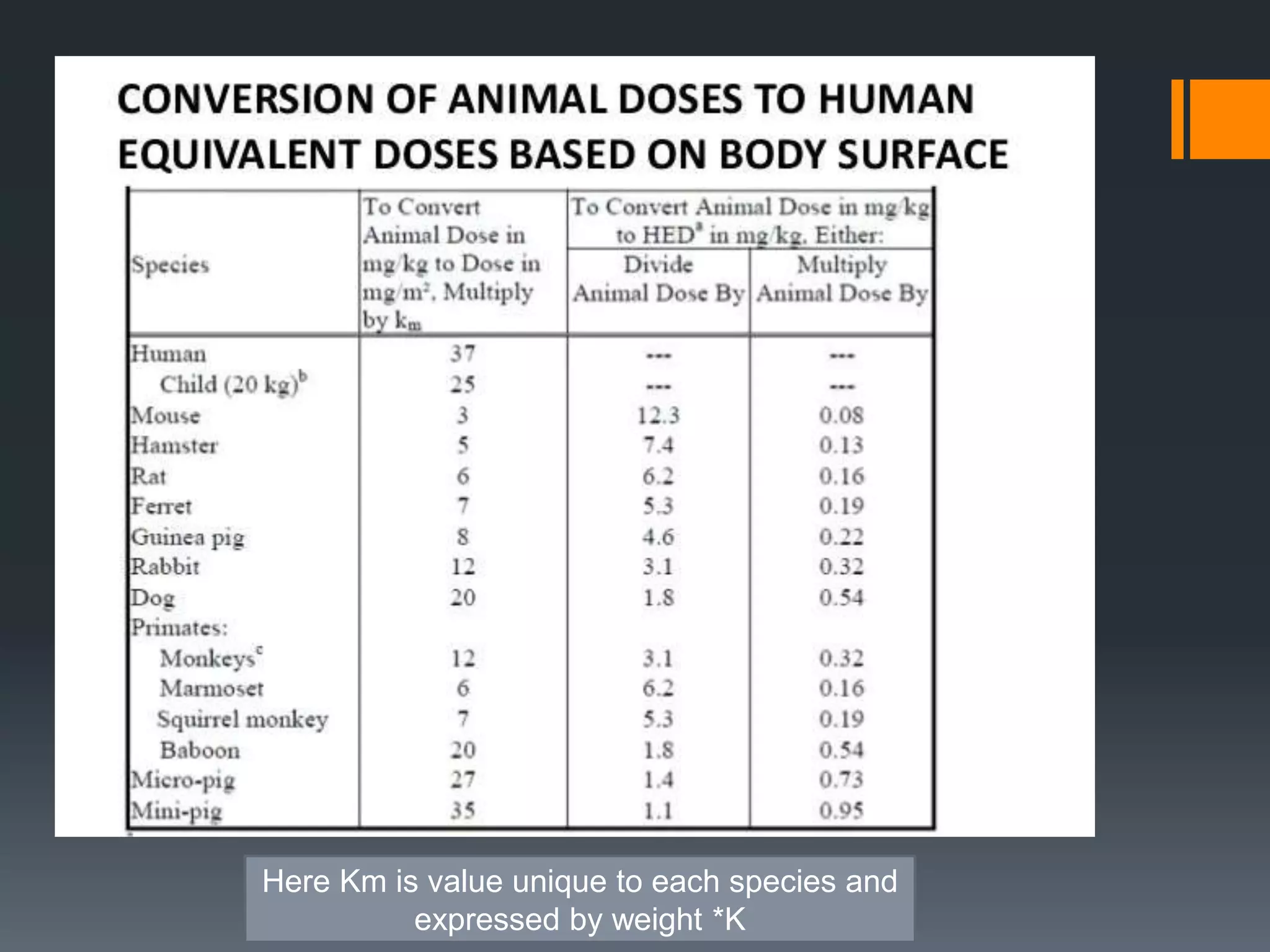
2) The no-observed-adverse-effect level (NOAEL) from animal studies is used to calculate a human equivalent dose (HED) based on body surface area, accounting for differences between species.
3) Additional safety factors are applied