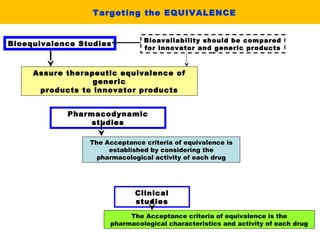
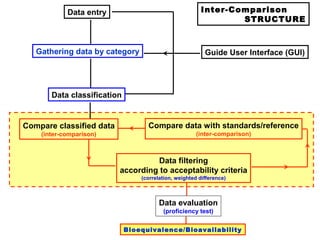
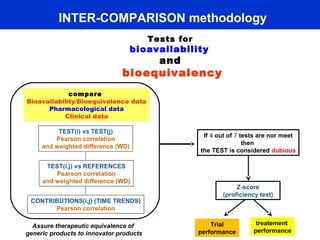
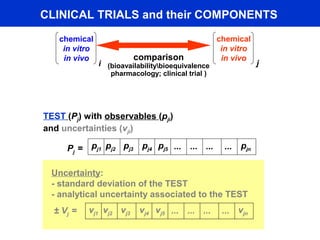
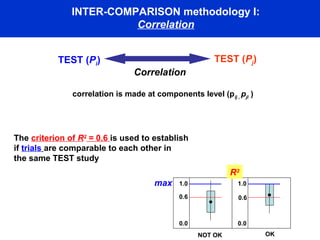
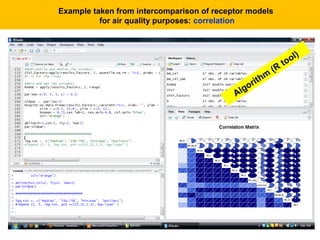
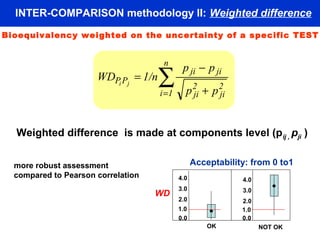
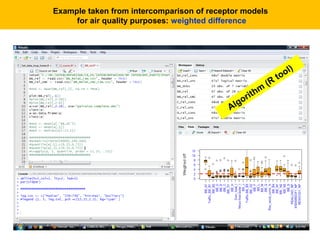
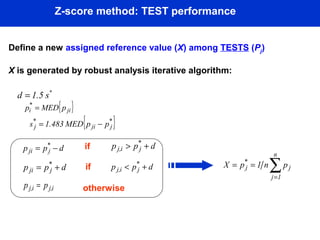
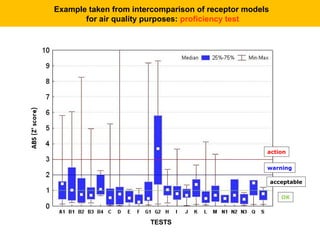
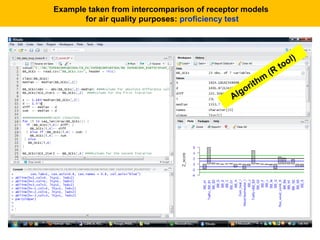
This document describes a methodology for comparing bioavailability and bioequivalence data from pharmaceutical clinical trials. The methodology involves gathering generic and innovator drug data, classifying the data, comparing the data using statistical methods, and establishing equivalence criteria. The comparison methods calculate correlation coefficients and weighted differences between tests, and use z-scores to evaluate test performance in a proficiency test. The goal is to assure therapeutic equivalence between generic and innovator drugs.