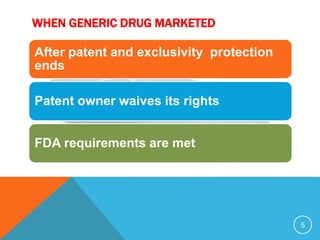
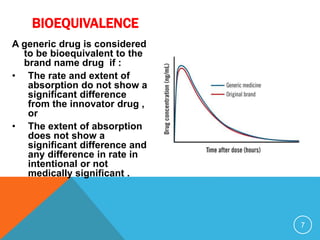
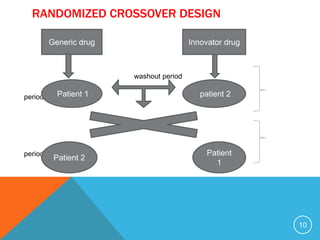
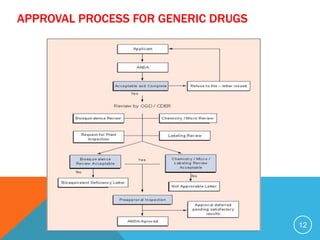
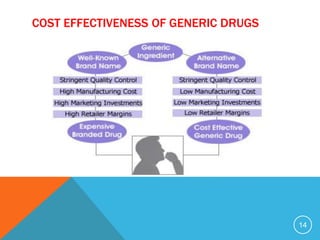
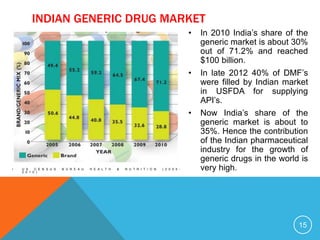
The document discusses generic drugs, including their regulatory approval process. Generic drugs must demonstrate bioequivalence to the branded version to gain approval. They are approved through an abbreviated new drug application that shows they deliver the same amount of active ingredients as the branded drug. India has a large and growing generic drug industry that supplies over 30% of the global generic market. Recent FDA rules now require generic drug makers to pay user fees for product approvals.