This document provides instructions and materials for an assignment on acids and bases. It includes:
1. A list of materials needed for the assignment, including notes on acids and bases and chemistry of living things.
2. Instructions to log into LearningPoint and lead an online discussion by highlighting information and answering questions about ions, the pH scale, acids, bases, and their characteristics and uses.
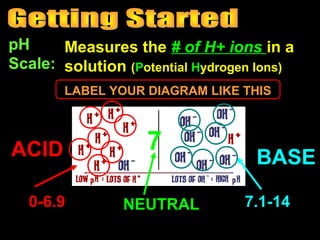
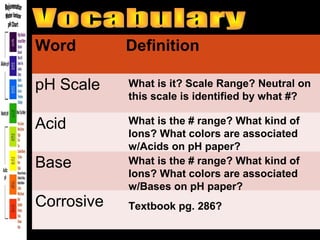
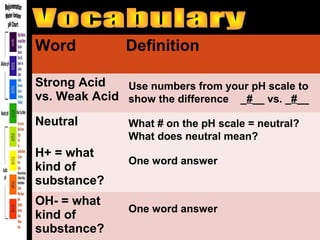
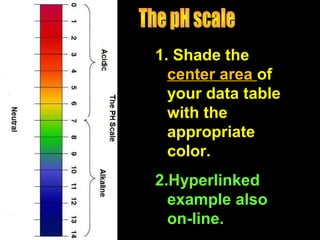
3. A data table to complete with definitions and examples related to pH, acids, bases, and their properties.
The document provides resources and guidance to study acids and bases, complete assignment questions, and participate in an online discussion on the topic.