Embed presentation
Download to read offline

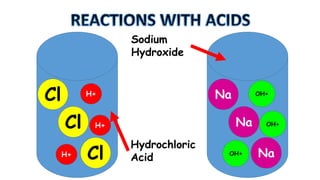
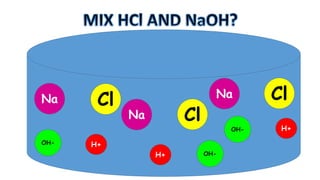
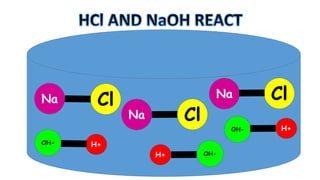
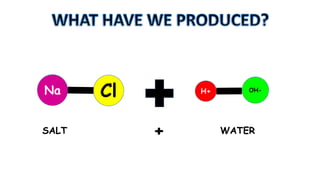



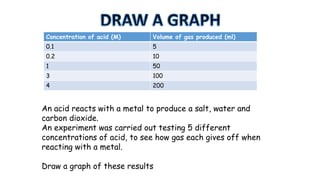


The document outlines the learning goals related to neutralization, reactions of acids with metals and carbonates, and a series of experiments to observe gas production from different acid concentrations. It describes the importance of identifying unknown liquids using a plant indicator method to distinguish between an acid, a base, and water. The overall objective is to understand the chemical reactions and safety considerations involved.









