Understanding p h and ph indicators: for class 6
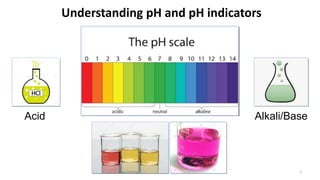
- 1. Understanding pH and pH indicators Acid Alkali/Base 1
- 2. What is pH? • pH is a number indicating acidity or alkalinity of an aqueous solution – Introduced by Danish Chemist Søren Peder Lauritz Sørensen in 1909 – He worked in Carlsberg Laboratory, Denmark – The ‘H’ in pH stands for ‘Hydrogen’ – What about ‘p’? • German chemists think ‘p’ for ‘Potenz (power)’ • French chemists think ‘p’ for ‘Puissance (power)’ 2 Carlsberg Foundation believes pH stands for ‘Power of Hydrogen’
- 3. pH of some solutions in our daily life 3
- 4. • pH plays important role in everyday life – pH (7.0 – 7.8) is essential for ideal growth/development of human body – pH of 1 – 3 is essential in stomach to activate an enzyme pepsin responsible for digestion – pH of 6.5 – 7.3 is essential for growth of plants and crops – pH in mouth should be around 7 to prevent tooth decay – Insects use chemical warfare for self-defence • Ants and honey bees inject powerful and skin irritating acids for self-defence 4 Why pH is important?
- 5. How to test pH of a solution? • Litmus paper test – Litmus is a dye obtained from lichen (a slow growing plant) 5 Rocella Tinctoria Lecanora tartarea
- 6. How litmus test work? • Litmus is a Dye – Dyes are colored compounds – The color of the dye depend on its structure • Structure? – Arrangement of objects in a particular way 6
- 7. • Structure of molecule/compound – Arrangement of different atoms in a molecule/compound 7 O HH H2O (water) C C O O H H H H CH3COOH (Acetic acid) Chlorophyll (Green) Notice so many are molecules made with different arrangement of atoms of ‘C’, ‘H’ & ‘O’
- 8. 8 Litmus dye Purple color Acid RED Litmus H + - BLUE Litmus So..how do you make BLUE litmus paper and RED litmus paper?
- 9. • Acid-Base indicator solutions – They are compounds which show different color with acids and bases Examples: • Phenolphthalein Acid (colorless) Base (pink) • Methyl orange Acid (Red) Base (yellow) 9 Color change of the indicator in acidic and basic solution is due to change in structure of the indicator compound
- 10. • Phenolphthalein 10 Acid (colorless) Base(Pink) Acid (RED) Base(Yellow) • Methyl orange
- 11. Calculating the pH • pH is a number indicating acidity or alkalinity of a solution • pH of a solution is related to the concentration of hydrogen ions • Concentration of a substance – It is a measure of amount of that substance in solution 11800 gram of sugar in 400 mL of water 80 gram of sugar in 80 mL of water 800 g/400 mL 2 g/mL 2000 g/L 2 kg/L 80 g/80 mL 1 g/mL 1000 g/L 1 kg/L
- 12. 12 1 kg 1 kg1 kg Will the number of fruits be the same in all baskets? NO
- 13. 10 g H2SO4 13 10 g Sugar (C12H22O11) 10 g Salt (NaCl) 10 g NaOH 10 g Water Will the number of molecules be the same in each case? NO 10 g HCl
- 14. Amount in terms of number • Dozen 14 12 numbers 12 numbers12 numbers Will a dozen apples, oranges and mangos have the same weight? NO
- 15. Mole • Mole is another unit for amount in terms of number • Mole is similar to the dozen unit • 1 dozen = 12 numbers •BUT • 1 Mole = 6.023 x 100000000000000000000000 items =6.023 x 1023 items 15 The number 6.023 x 1023 is called Avagadro’s number
- 16. • 1 Mole of H2O 6.023 x 1023 molecules of H2O • 1 Mole of He (gas) 6.023 x 1023 atoms of He • 1 Mole of Sugar 6.023 x 1023 molecules sugar (C12H22O11) • 1 Mole of HCl 6.023 x 1023 molecules of HCl • 1 Mole of HCl atoms of H • 1 Mole of HCl atoms of Cl • 1 Mole of H2SO4 Mole of H atoms, Mole of S atoms, Moles of O atoms 16 6.023 x 1023 6.023 x 1023 2 1 4
- 17. Concentration of solutions • Concentration – Number of moles of a substance in one litre of solution • If you dissolved 1 Mole of HCl in 1 L of water you will get HCl solution of concentration 1 mole/L • So how many HCl molecules will be there in 1 litre of HCl solution having concentration 1 mole/L? 17 6.023 x 1023 molecules of HCl
- 18. Calculating the pH • pH is a number indicating acidity or alkalinity of a solution • pH of a solution is related to the concentration of hydrogen ions • Ion – An atom or a molecule with charge • Dissociation – Splitting of molecules into ions 18 H+ Cl - H+ Cl - H+ Cl - 1 mole HCl 1 mole H+ 1 mole Cl -
- 19. HCl H+ + Cl- H2SO4 2H+ + SO4 2- KOH K+ + OH- NaOH Na+ + OH- 19 H+ OH - H+ OH - 1 Litre of water • concentration of H+ ions = 0.0000001 moles/L = 10-7 moles/L • In water, concentration of H+ ions and concentration of OH- ions are equal In acidic solution we have more of H+ ions In basic solution we have more of OH- ions
- 20. 20 Concentration of H+ (moles/L) Concentration of OH- (moles/L) pH Acidic 1 - 0 Acidic 0.1 (10-1) - 1 Acidic 0.01(10-2) - 2 Acidic 0.001 (10-3) - 3 Acidic 0.0001 (10-4) - 4 Acidic 0.00001 (10-5) - 5 Acidic 0.000001 (10-6) - 6 Water (Neutral pH) 0.0000001 (10-7) 0.0000001 (10-7) 7 Basic - 0.000001 (10-6) 8 Basic - 0.00001 (10-5) 9 Basic - 0.0001 (10-4) 10 Basic - 0.001 (10-3) 11 Basic - 0.01 (10-2) 12 Basic - 0.1 (10-1) 13 Basic - 1 14 HCl concentration = 0.1 moles/L pH = 1 HCl concentration = 0.5 moles/L pH between 0 and 1 NaOH concentration = 1 moles/L pH = 14
- 21. Universal indicator • A combination of several indicators which can cover pH from 0 to 14 21
- 22. Make your own pH indicator at home 22 Red/Violet cabbage chop cabbage boil cabbage Separate the juice