Embed presentation
Download to read offline



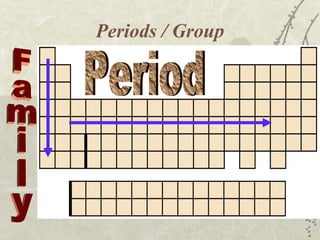



The periodic table arranges elements in order of increasing atomic number, with each row representing a period and each column a group or family. Periodic law states that physical and chemical properties repeat periodically when elements are arranged this way. Properties vary systematically across periods, with elements becoming less metallic and more nonmetallic from left to right, ranging from the most reactive metal to the most reactive nonmetal.






