Embed presentation
Download to read offline


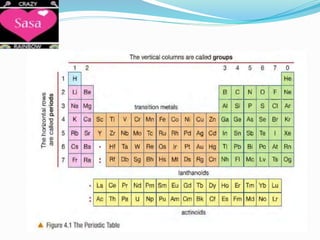
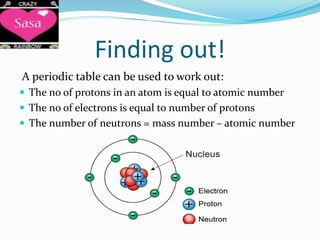


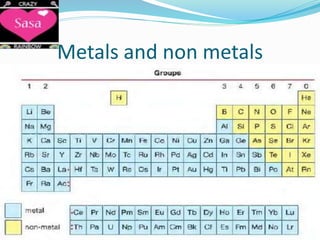

The periodic table arranges elements in order of increasing atomic number and groups elements with similar chemical and physical properties. Elements are organized into rows called periods and columns called groups. The periodic table can be used to determine the number of protons, electrons, and neutrons in an atom based on its atomic number and mass number. Elements in the same group have the same number of electrons in their outer shell, which is equal to the group number for groups 1 to 7. Noble gases make up group 0 and are unreactive because their outer shell is full.







