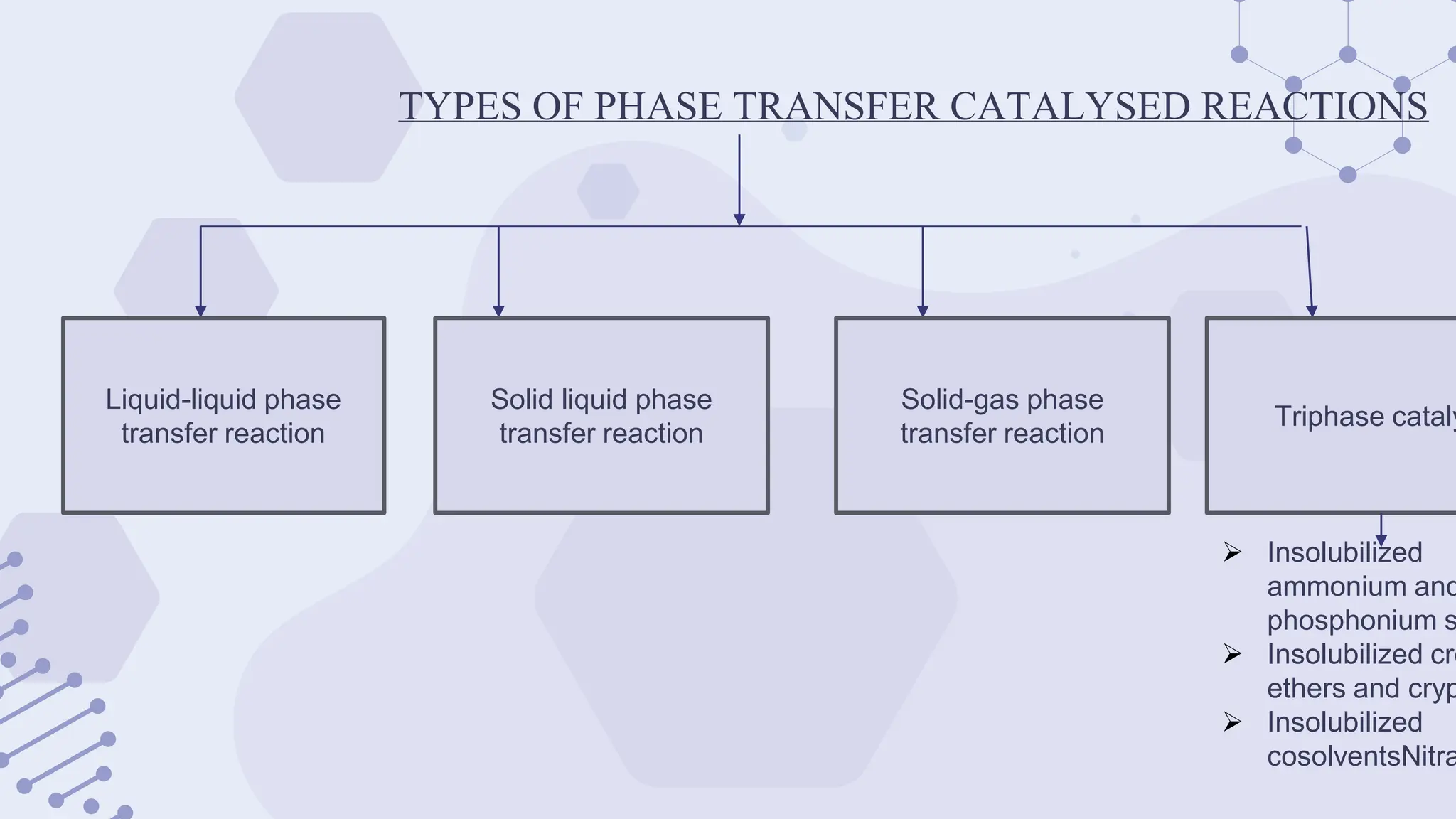
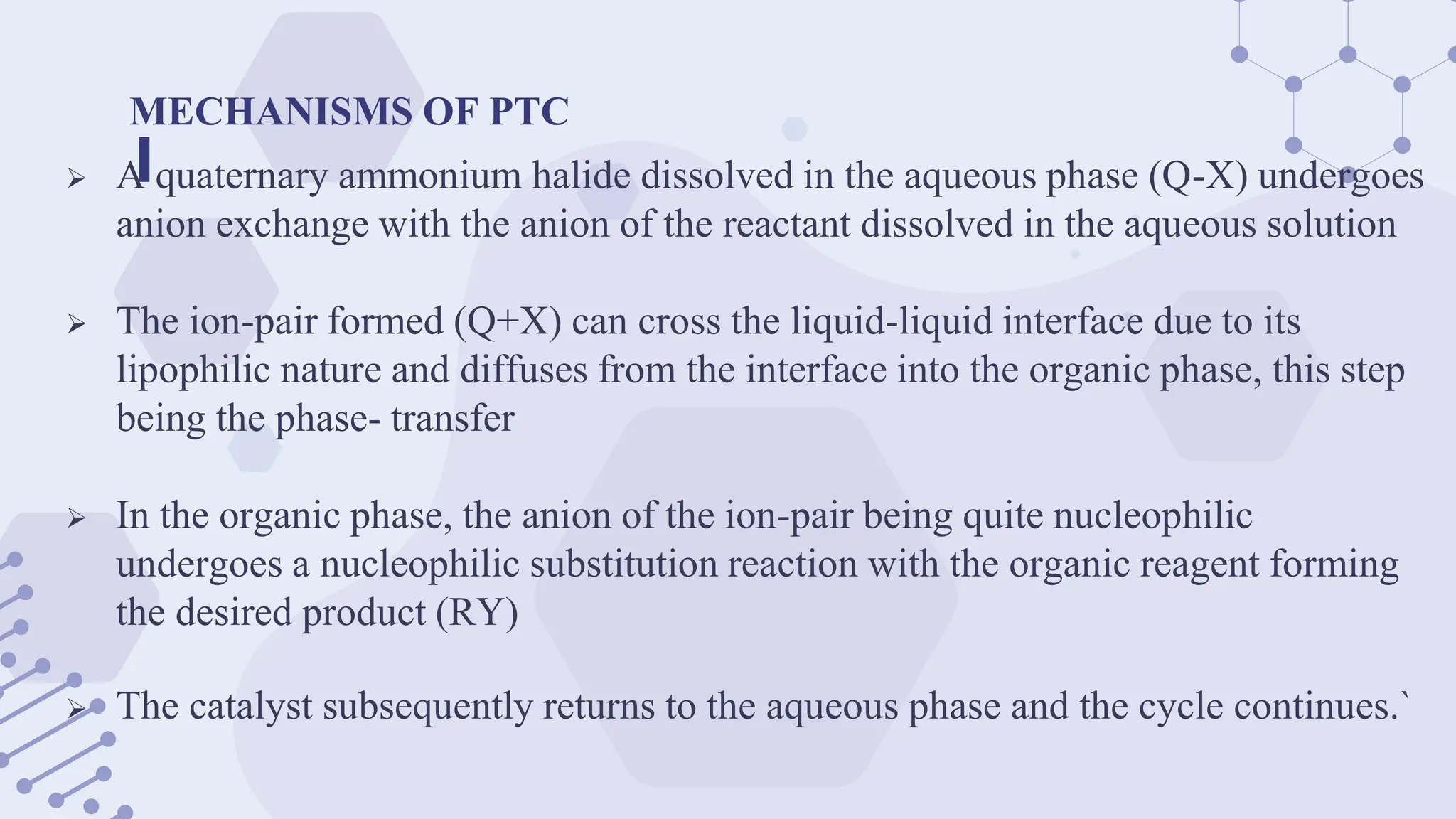
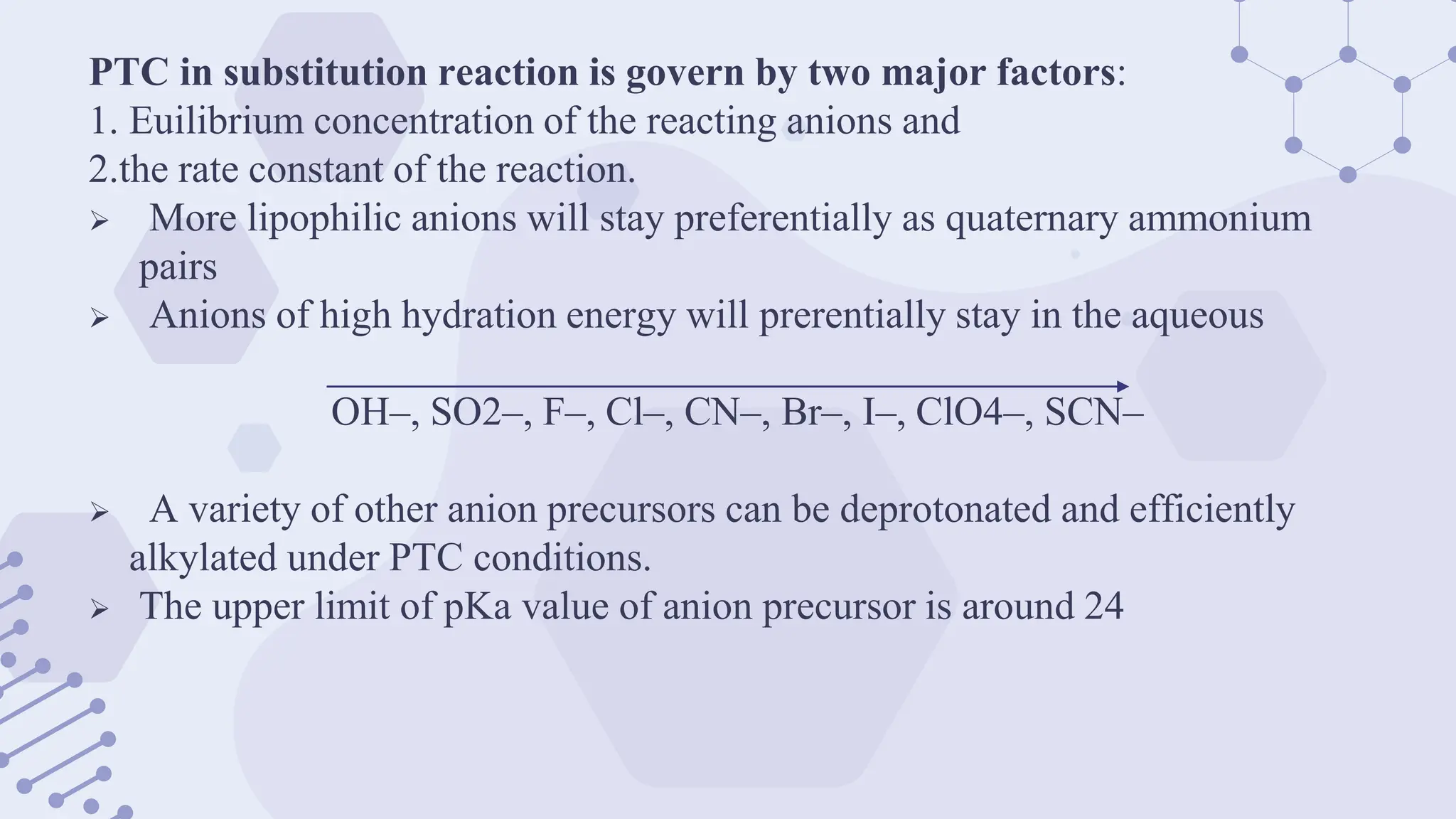
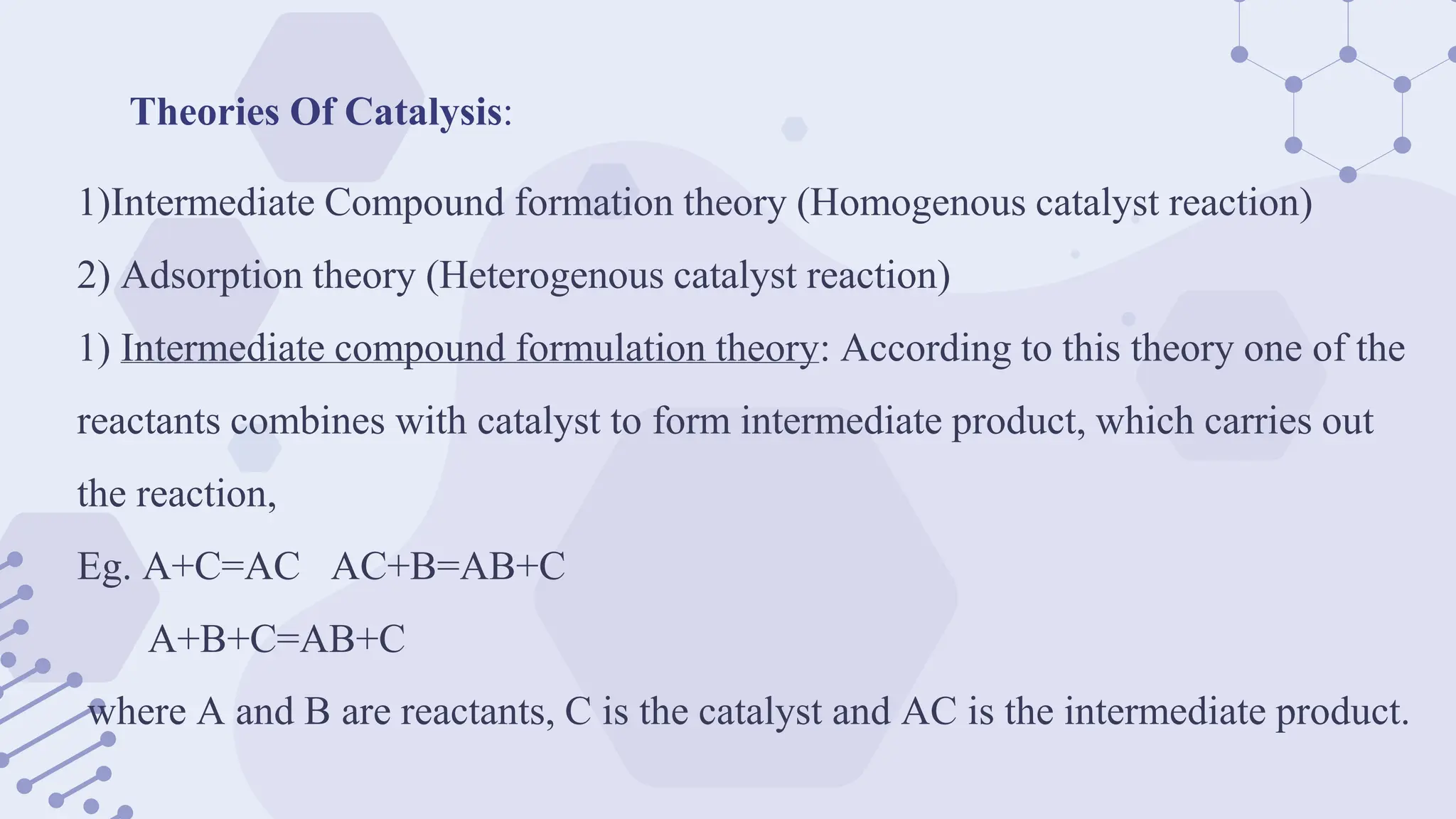
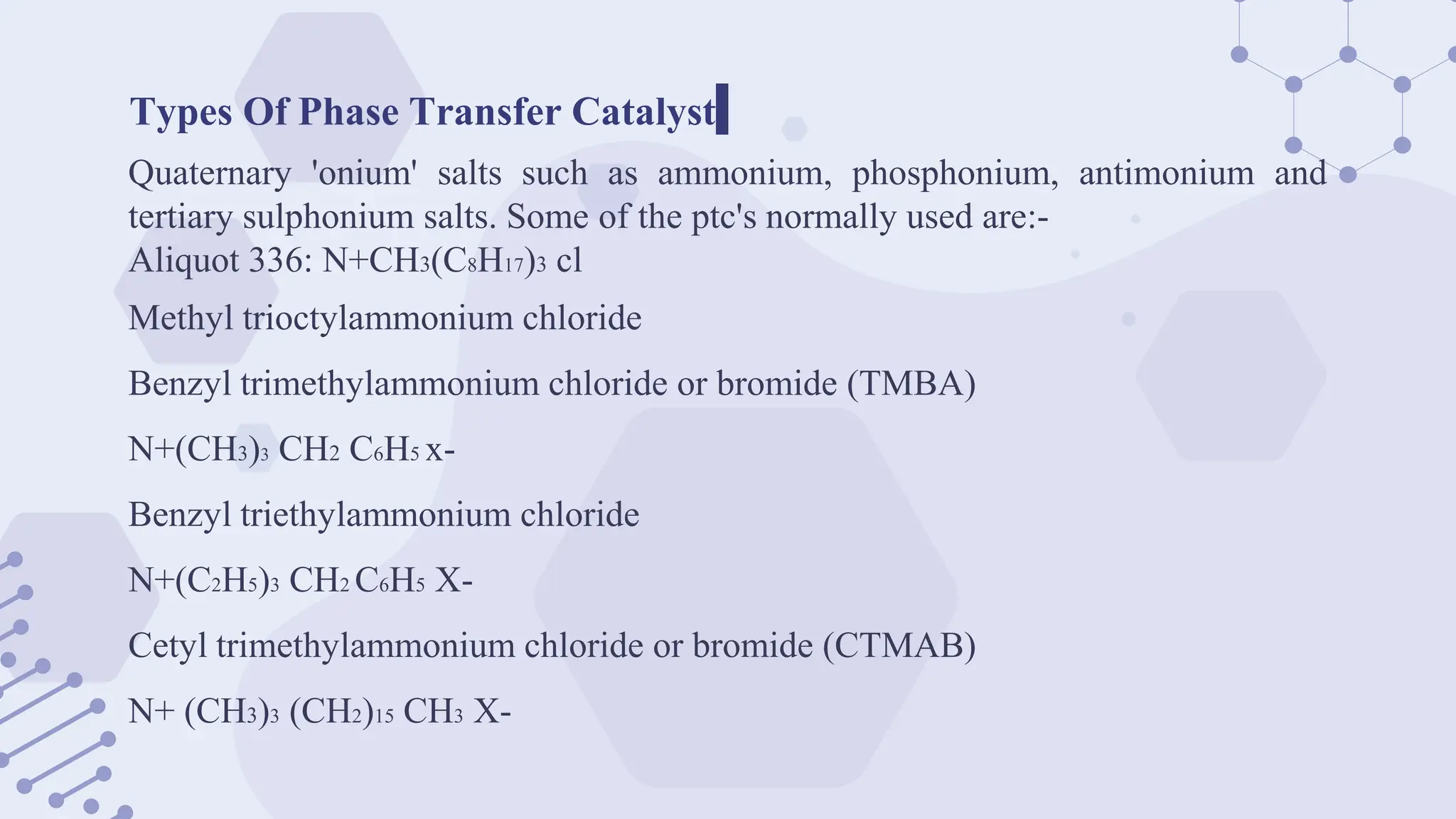
The document explores phase-transfer catalysis (PTC), a process that enhances the rate of chemical reactions by enabling the migration of reactants between different phases. It details the principles, mechanisms, types of PTC, and its applications in various industries, particularly in organic synthesis and pharmaceuticals. The advantages include mild reaction conditions and greater accessibility of reactants, while the main challenges involve catalyst separation from products.
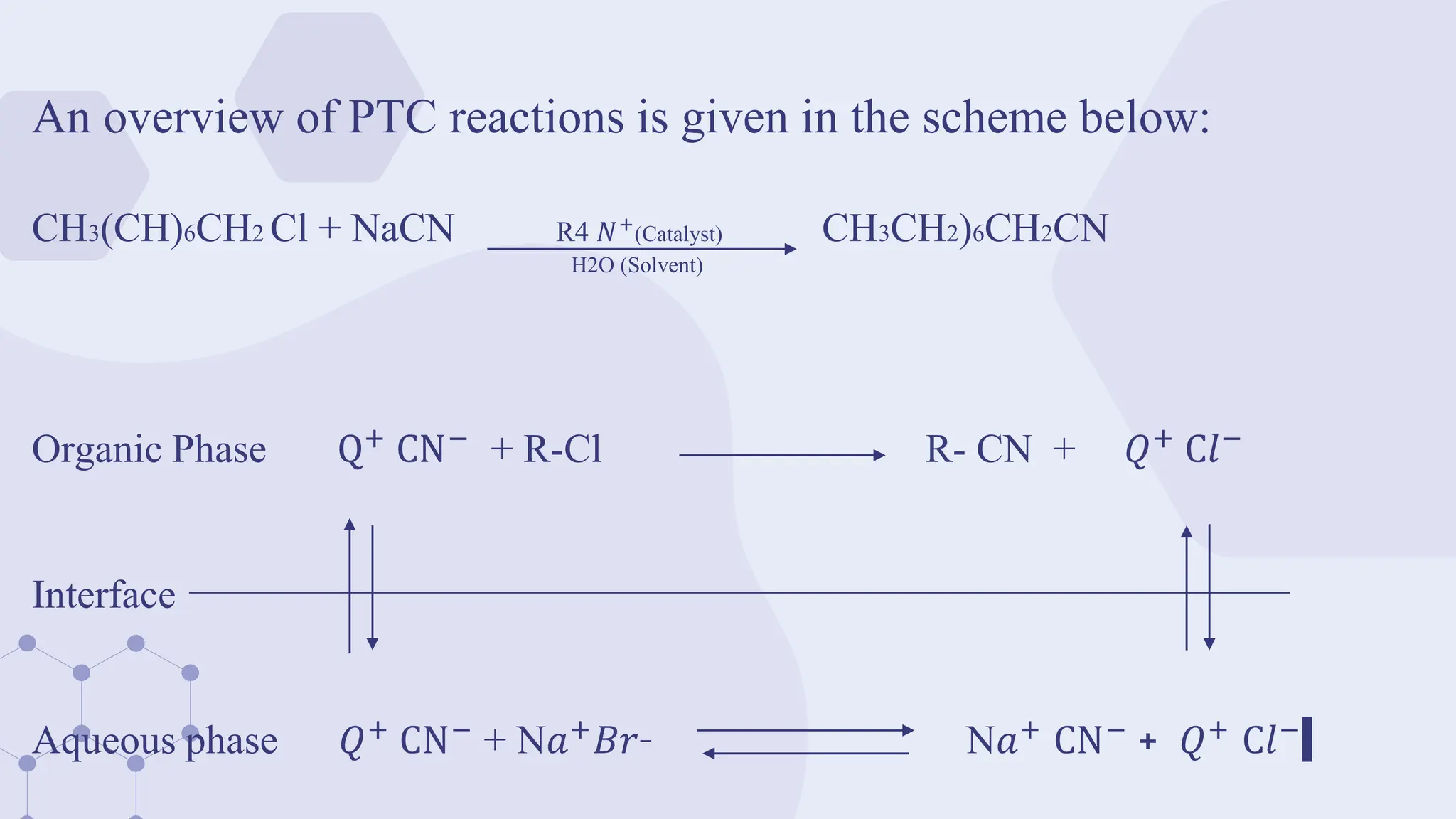
![In 1971, Starks introduced Phase Transfer Catalysis (PTC) to explain the role of
tetraalkylammonium or phosphonium salts in the reactions between two substances
located in different immiscible phases.
e.g. the displacement reaction of 1-chlorooctane with aqueous sodium cyanide is
accelerated > 1000x by the addition of hexadecyltributylphosphonium bromide as a
PTC.
[CH3(CH2)15𝑃+
(n-Bu)3]
C8H17Cl C8H17CN
1-Chlorooctane NaCN, H₂O, 105 °C. 1.8 h 1-Cynooctane](https://image.slidesharecdn.com/phasetransfercatalysisptc-240412110407-56d3a359/75/Phase-transfer-catalysis-Theory-and-application-5-2048.jpg)