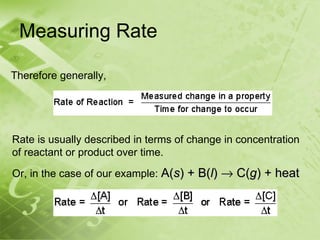
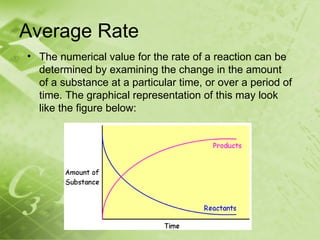
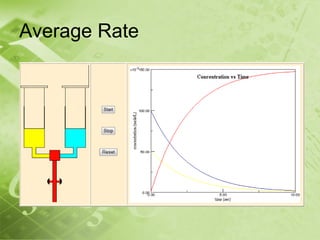
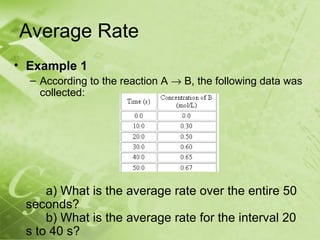
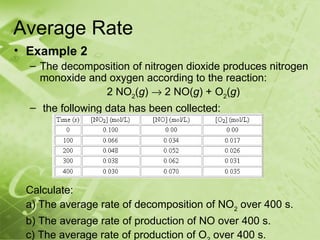
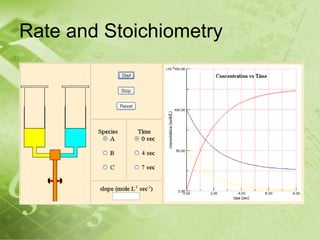
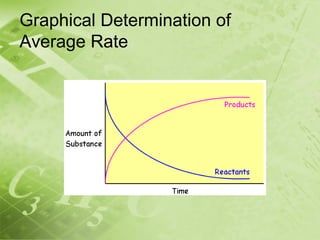
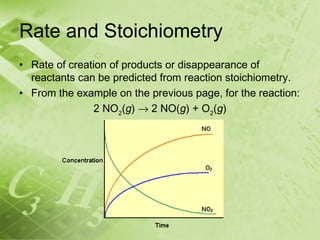
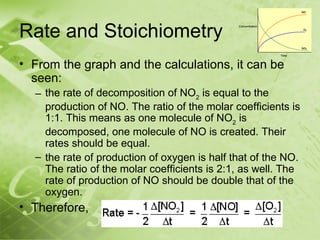
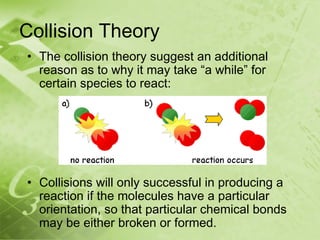
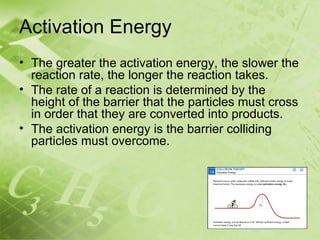
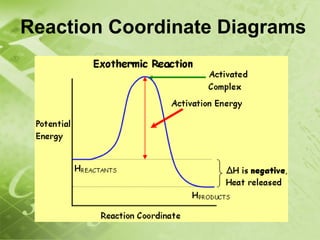
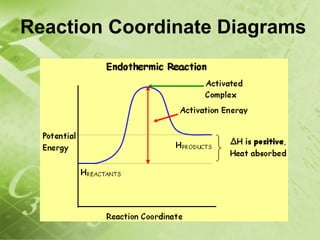
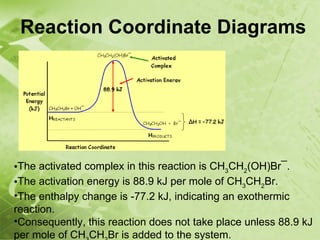
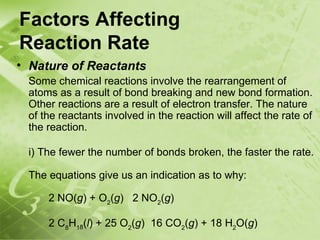
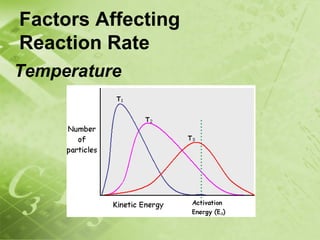
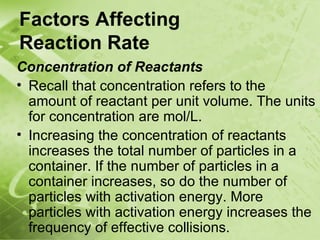
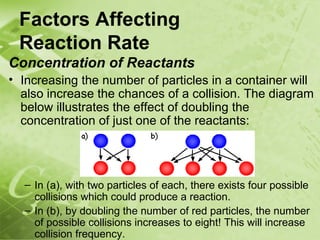
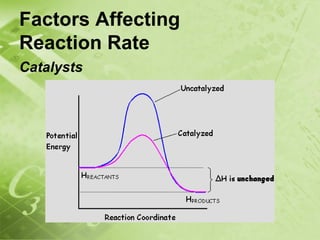
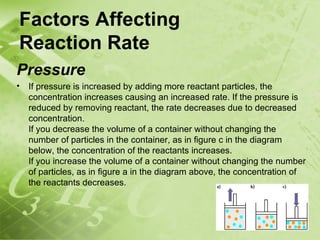
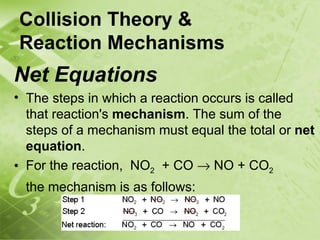
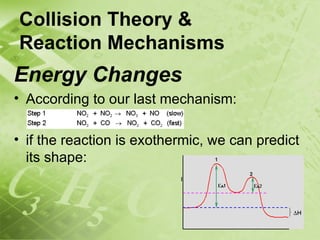
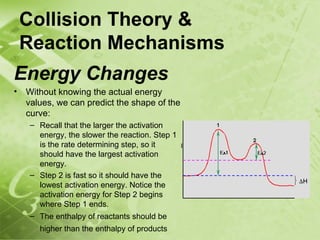
The document discusses kinetics and reaction rates. It defines kinetics as the branch of chemistry that studies the speed or rate of chemical reactions. It explains that reaction rates can be measured by changes in concentration, temperature, or pressure over time. The rate depends on factors like the nature of reactants, concentration, temperature, catalysts, surface area, and pressure. Reactions may occur in multiple steps through reaction intermediates rather than a single step. The collision theory and concept of activation energy are introduced to explain why certain collisions result in reactions. Reaction coordinate diagrams are used to illustrate the energy changes in reactions.


































![Rate Law
The Molarity Relationship
• M = n,
V
• the molar concentration α number of moles of substance
– therefore R α [A] and R α [B]
– AND: R α [A]x [B]y AND: R = k [A]x [B]y
• R = rate of reaction (Moles/1/s or M/s)
• [A] = molar concentration of A
• [B] = molar concentration of B
• k = specific rate constant
• x, y = is the power, called the order of the reaction](https://image.slidesharecdn.com/chem30sunit2-2010-120611125255-phpapp01/85/Chem-40S-Unit-3-Notes-62-320.jpg)
![Rate Law
The Molarity Relationship
• R = k [A]x [B]y
• The constant k, is known as the specific rate constant
for the reaction. The rate constant and the order can
only be determined experimentally.
• The rate constant is specific for each reaction at a
specific temperature, since it’s value depends upon the
size, speed and types of molecules in the reaction.
• Changing temperature would change the speed of the
reactant particles and hence change the rate constant.
• Temperature is the only factor, which affects the rate
constant.](https://image.slidesharecdn.com/chem30sunit2-2010-120611125255-phpapp01/85/Chem-40S-Unit-3-Notes-63-320.jpg)



![Rate Law
Reaction Order
• For a reaction with more than one reactant, such as
A + B → products
• The rate law would be: Rate = k[A]x[B]y
• The rate depends on both A and B concentrations.
Each reactant can affect the rate differently.
• The total order of the reaction is the sum of the
order with respect to A and the order with respect to
B, x + y.](https://image.slidesharecdn.com/chem30sunit2-2010-120611125255-phpapp01/85/Chem-40S-Unit-3-Notes-67-320.jpg)

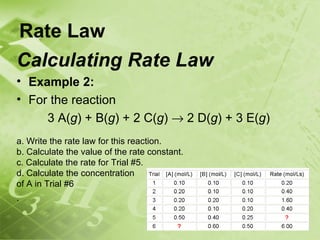
![Rate Law
Rate Law & Stoichiometry
• For most reactions, the rate law, the specific rate constant,
k, and the mechanism of a reaction can only be determined
experimentally, not from the reaction stoichiometry.
• However, for reactions that occur in a single step
(elementary reactions) the order of each reactant in the
rate law is equal to the coefficient in the reaction's
balanced equation.
• For the elementary reaction:
aA + bB → cC + dD
• the rate law is
rate = k[A]a[B]b
• where a and b are the molar coefficients for the elementary
reaction.](https://image.slidesharecdn.com/chem30sunit2-2010-120611125255-phpapp01/85/Chem-40S-Unit-3-Notes-71-320.jpg)

![Rate Law
Rate Law & Reaction Mechanism
• Example
• The mechanism for the reaction
3M + N → P + 2Q
• is below:
2M → P + X (slow)
X+M→ Q + Y (moderate)
N+Y → Q (fast)
a) What is the rate law for this reaction?
b) What would be the effect of tripling the [M]?
c) What would be the effect of doubling the [N]?](https://image.slidesharecdn.com/chem30sunit2-2010-120611125255-phpapp01/85/Chem-40S-Unit-3-Notes-73-320.jpg)
