Embed presentation
Downloaded 37 times


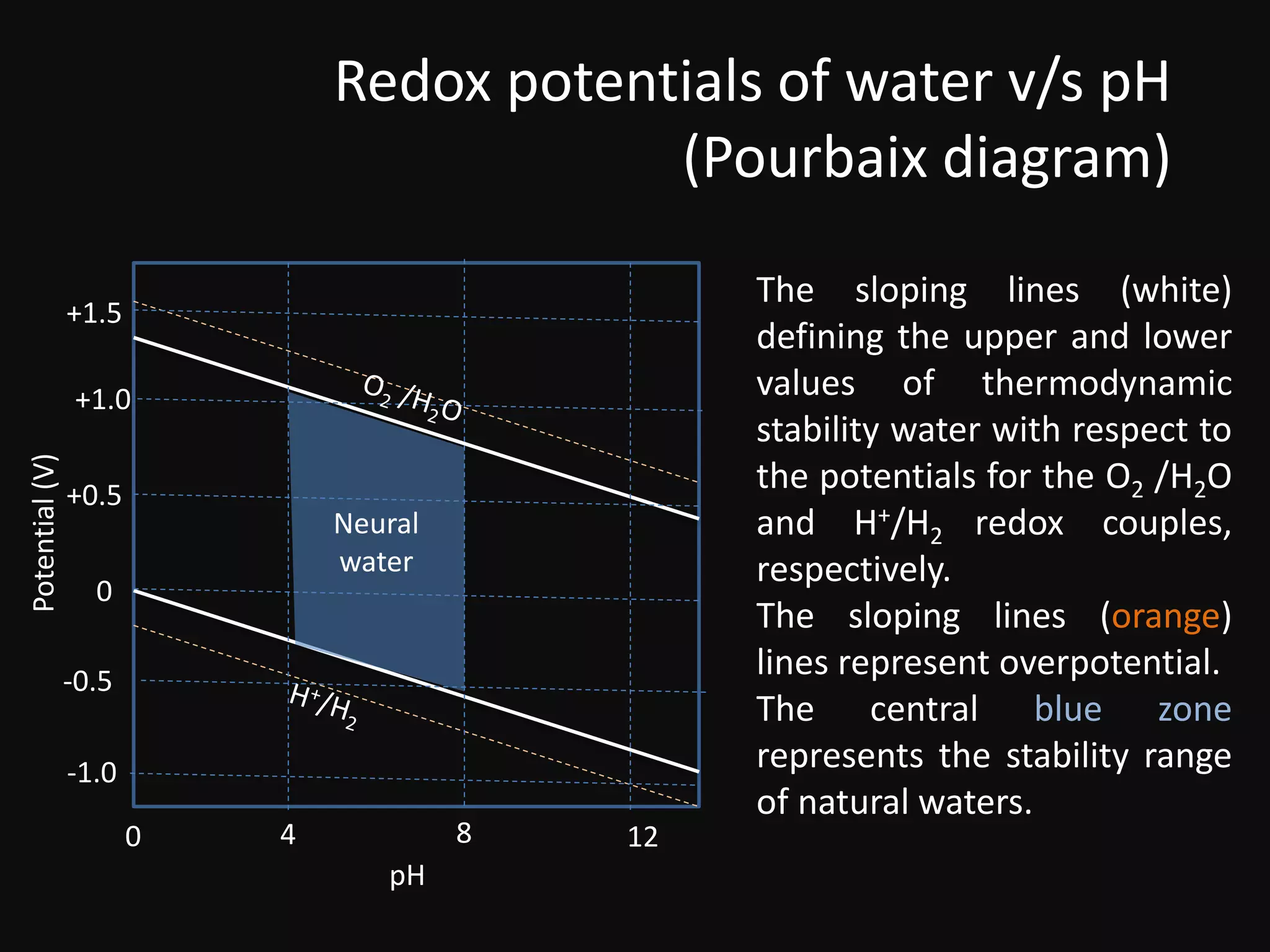

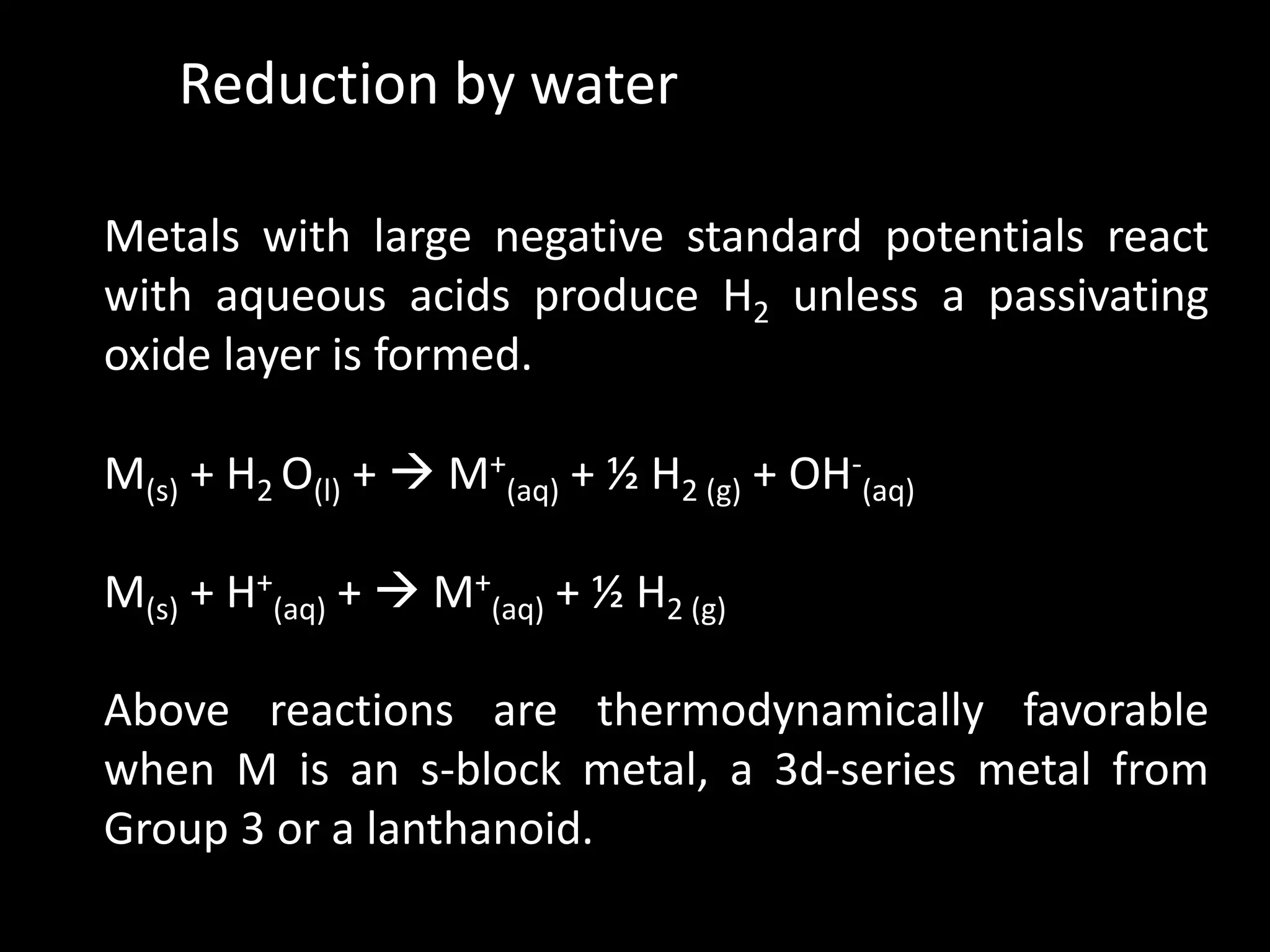
This document discusses the redox stability of water. It provides the redox potentials of water with respect to pH and defines the stability range of natural waters. It also discusses how water can act as both an oxidizing agent and reducing agent in redox reactions. For example, water is a poor reducing agent except towards strong oxidizing agents. However, metals with large negative standard potentials can reduce water to produce hydrogen gas. Understanding the redox stability of water is important for generating hydrogen fuel from water.





