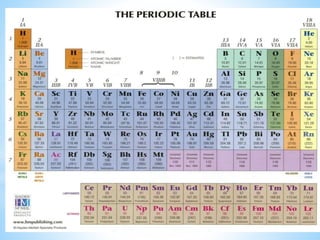
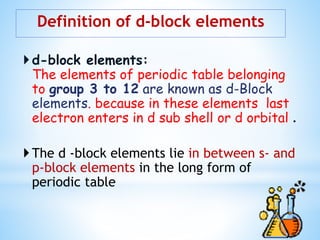
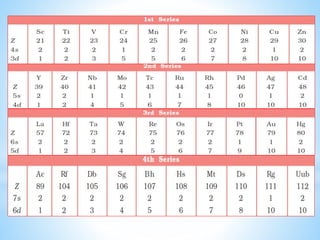
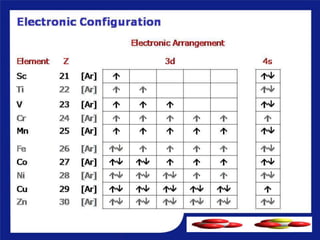
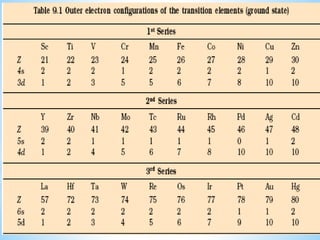
1) D-block elements are those whose last electron enters the d orbital, lying between s- and p-block elements.
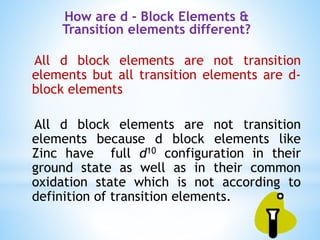
2) Not all d-block elements are transition elements, which are defined as having partially filled d orbitals, while all transition elements are d-block.
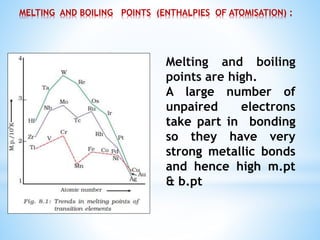
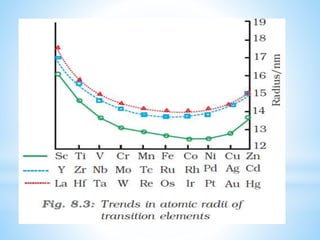
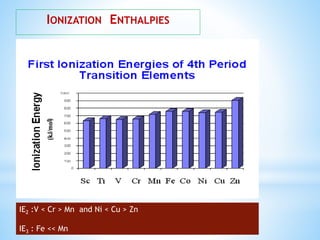
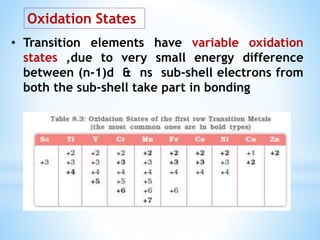
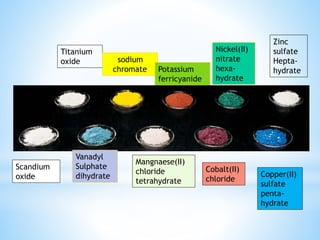
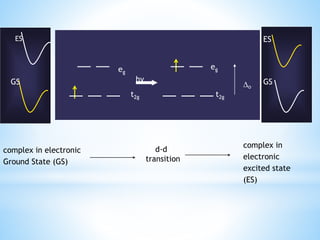
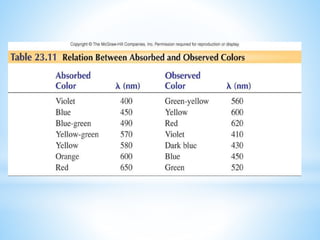
3) General properties of d-block elements include high melting/boiling points due to strong metallic bonds, variable oxidation states, and many forming colored ions or complexes.