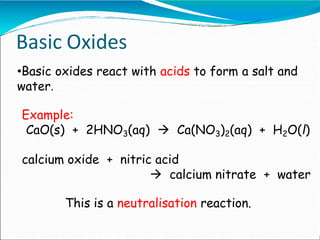
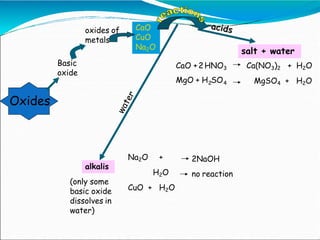
An oxide is a compound formed between oxygen and another element. There are four main types of oxides: acidic, basic, amphoteric, and neutral. Acidic oxides are nonmetals that form acids when dissolved in water. Basic oxides are metals that react with acids to form salts and water. Amphoteric oxides like zinc oxide react with both acids and bases to form salts and water. Neutral oxides show no acidic or basic properties and do not react with acids or bases.