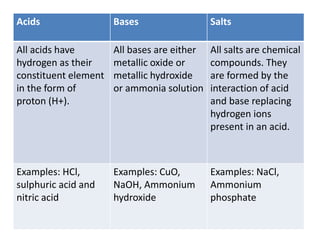
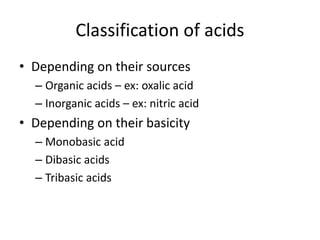
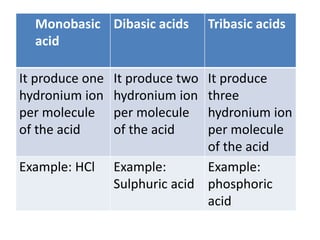
This document summarizes acids, bases, and salts. It states that acids have a sour taste and contain hydrogen ions, bases have a soapy taste and are usually metal oxides or hydroxides, and salts are formed from the reaction of acids and bases. It then provides more details on acids, including that they contain hydrogen and produce hydronium ions in water. It classifies acids as organic or inorganic and monobasic, dibasic, or tribasic based on the number of hydronium ions produced. Examples of common acids and their properties are also given.