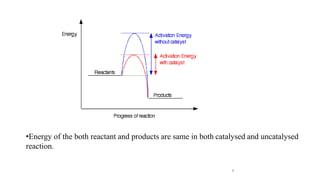
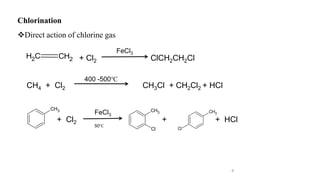
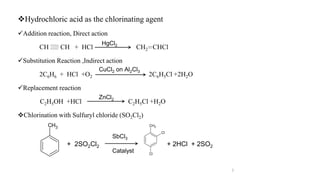
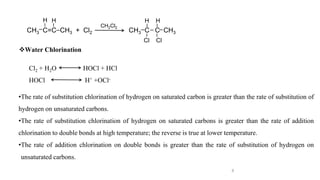
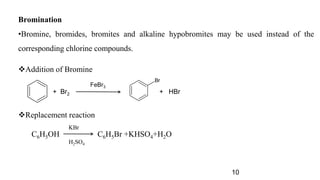
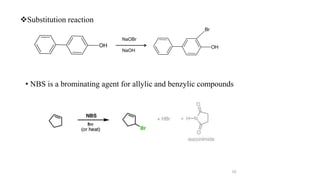
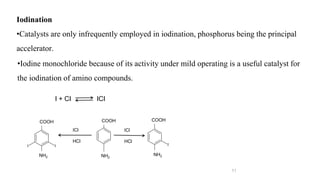
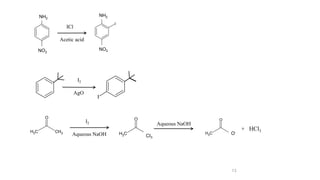
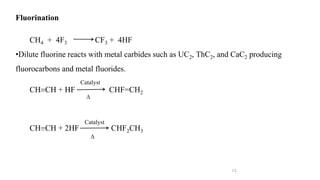
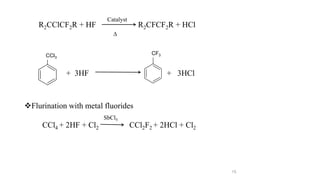
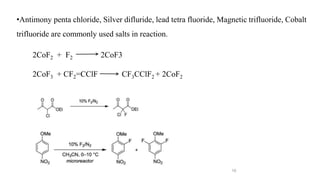
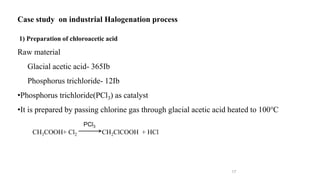
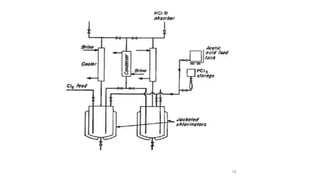
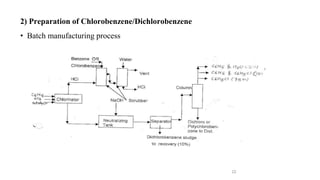
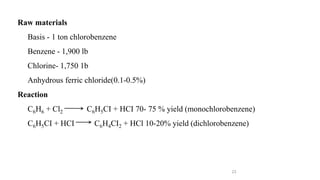
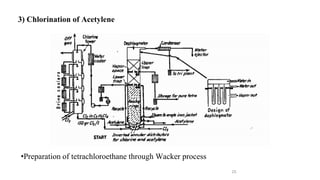
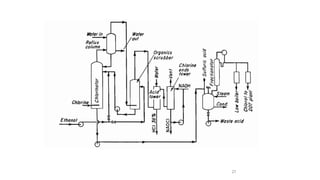
The document discusses various halogenation processes, including catalytic halogenations and specific case studies on industrial halogenation methods for producing compounds like chloroacetic acid and chlorobenzene. It details the chemical reactions involved, catalysts used, and the conditions required for these processes. Significant focus is placed on the specific industrial applications and the yields obtained from each method.