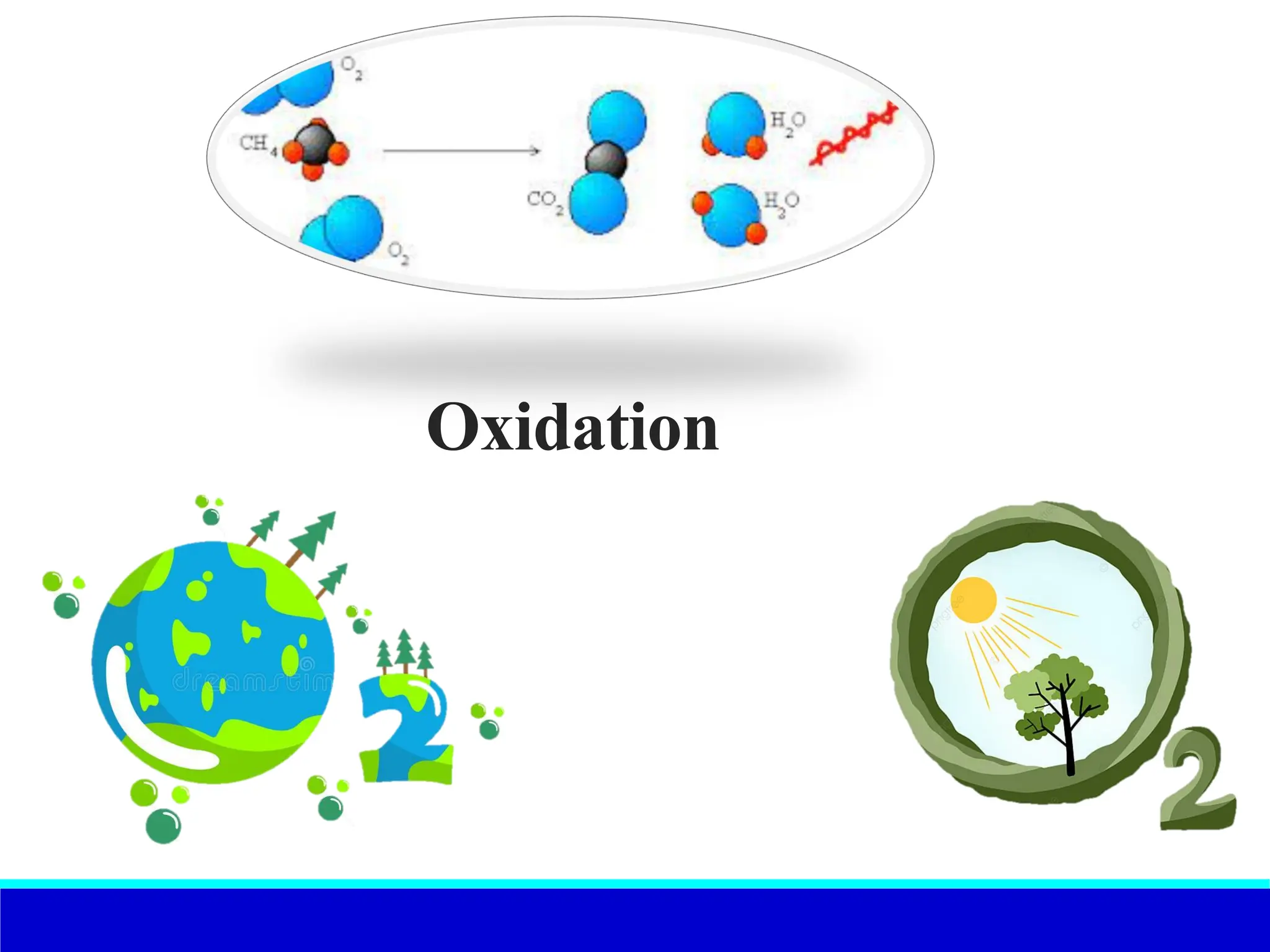
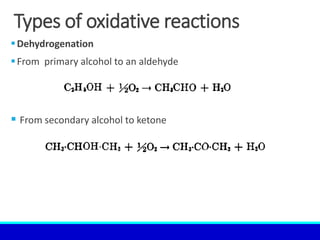
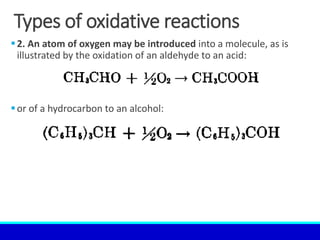
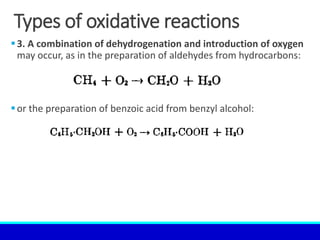
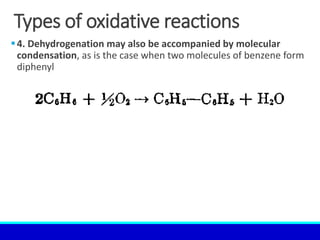
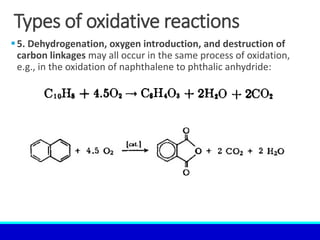
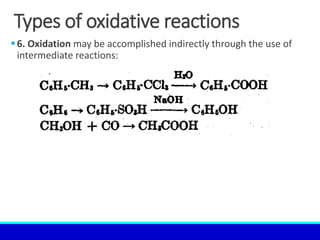
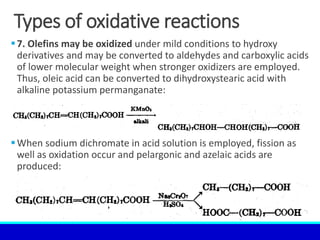
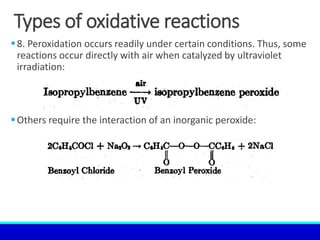
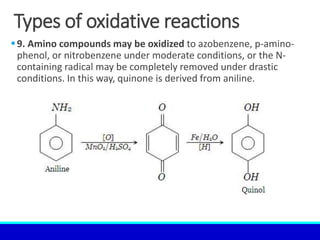
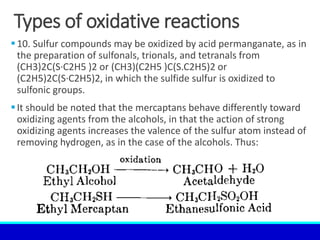
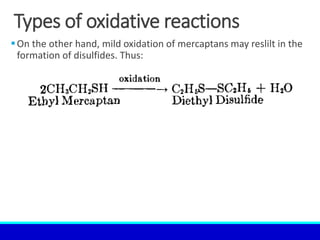
The document explains the concept of oxidation, describing various definitions and applications, including its role in photosynthesis, respiration, combustion, and corrosion. It emphasizes the significance of oxidation reactions in organic synthesis and wastewater treatment, as well as outlines different types of oxidative reactions and common oxidizing agents. Additionally, it discusses the kinetics and thermochemistry related to oxidation processes.